Exam Prep
JEE Main 2022: NTA Did Not Drop These Questions But They Were Wrong; Here’s Why

Synopsis
The JEE Main 2022 provisional answer keys for the June session caused a furore when the NTA published them. Even in the final JEE answer keys, many errors in the Chemistry questions were not addressed. Careers360 looks at some of the questions and the problems with them.

Synopsis
The JEE Main 2022 provisional answer keys for the June session caused a furore when the NTA published them. Even in the final JEE answer keys, many errors in the Chemistry questions were not addressed. Careers360 looks at some of the questions and the problems with them.
The Joint Entrance Examination (JEE) Main is the entrance test for admission into the National Institutes of Technology (NIT), Indian Institutes of Information Technology (IIIT) and many more government institutions and also acts as a preliminary round for admission into the Indian Institutes of Technology (IIT).
The first phase of the JEE Main, 2022 was conducted by the National Testing Agency (NTA) in the late-June and over 12 shifts. This time as well there were questions that were ambiguous or the answers were factually incorrect. The NTA published the final JEE Main 2022 answer key on July 6, dropping four questions from the test but still retaining several that were wrong or ambiguous.
The preliminary answer keys uploaded by the NTA were wrong and created panic and outrage among students. Answer keys for the exams held on June 29 which were incorrectly uploaded, have now been rectified. While a lot of the mistakes have been corrected, some ambiguities and errors still remain that have not been addressed in the updated answer keys. This begs the question – is the examination really fair for candidates? Considering the high stakes involved in the JEE Main, even a few such errors in questions can leave students deeply demoralised and also impact their academic prospects and careers.
Here, Careers360 looks at some of the questions asked in the June 2022 session of JEE Main 2022 and highlights some of the errors in the Chemistry section.
JEE Main 2022: The Problems
The questions, along with the problems with them, are listed out below
JEE Main Exam: June 24, Shift 1
QID: 101658

Answer Given: 63
Problem: The mass of Nitrogen in the organic compound comes out as 0.245 g which is greater than the mass given for the organic compound (0.166 g). This is not possible. The question should be awarded as a bonus.
____________________________________________________________________________
JEE Main Exam: June 24, Shift 2
QID: 1373
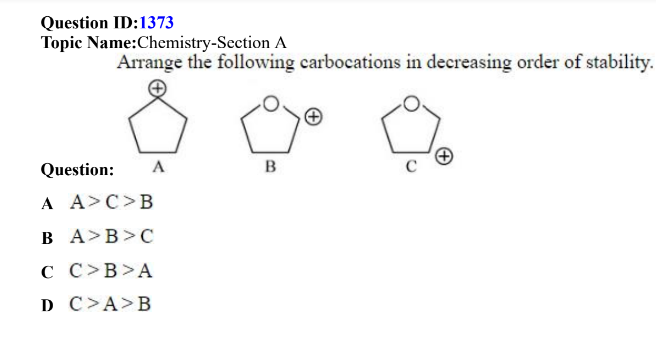
Answer Given: (B)
The Problem: The correct order of stability of the given carbocations is B>A>C.
The most stable carbocation is B. This can be explained on the basis of the +M effect exerted by the lone pairs of Oxygen.
The correct answer is not given as an option in the question and hence, the question should be awarded as a bonus
___________________________________________________________________________
JEE Main Exam: June 25, Shift 1
QID: 101448
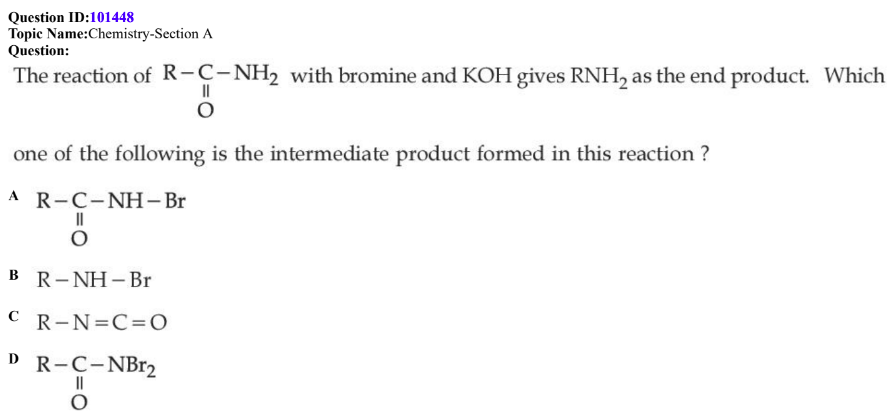
Answer Given: (C)
The Problem: In the Hoffman’s Bromamide Degradation reaction, the compounds in Option (A) and Option (C) are obtained as intermediates. Hence, both options (A) and (C) should be the answer.
____________________________________________________________________________
JEE Main Exam: June 25, Shift 1
QID: 101453

Answer Given: 4
The Problem: It is not possible to calculate the ionisation energy of the Li atom using the Bohr’s Model. Bohr’s model is only applicable to unielectronic species and since Lithium contains three electrons, the question is not solvable and should be awarded as a bonus.
____________________________________________________________________________
JEE Main Exam: June 26, Shift 2
QID: 1875
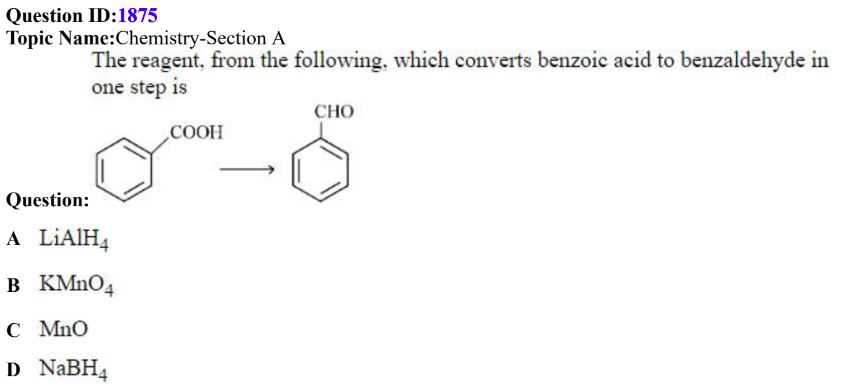
Answer Given: (C)
The Problem: When Benzoic acid reacts with MnO, the product formed is Benzophenone and not Benzaldehyde.
In order to obtain Benzaldehyde, we have to heat a mixture of Benzoic acid and Formic acid in the presence of MnO.
The language of the question is ambiguous and it does not indicate if any other reagent can also be used in the single-step conversion.
Benzoic acid cannot be converted into Benzaldehyde using MnO alone and hence, the question should be awarded as a bonus.
____________________________________________________________________________
JEE Main Exam: June 26, Shift 2
QID: 1883
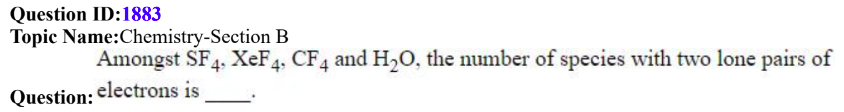
Answer Given: 2
The Problem: The given answer is 2 which will be correct when considering the lone pairs on the central atom only H2O and XeF4. The question does not specify that only the lone pair on the central atom has to be considered.
Now, if the other two compounds are considered, they too have more than two lone pairs of electrons on the fluorine atoms
So the answer could be any one of 1, 2 or 4 basis the assumptions below:
(i) Only H2O contains exactly 2 lone pairs of electrons
(ii) Both H2O and XeF4 contain 2 lone pairs of electrons on the central atom
(iii) All four of the given compounds have 2 or more lone pairs.
____________________________________________________________________________
JEE Main Exam: June 27, Shift 2
QID: 1987
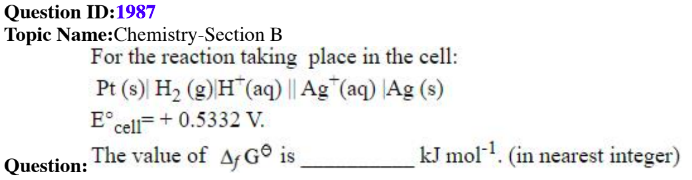
Answer Given : 51
The Problem: Change in Gibbs free energy is an extensive property which depends on the way the reaction is balanced.
Using different stoichiometric coefficients for balancing would lead to different values of the change in the Gibbs free energy and since the reaction is not given explicitly in the question, it becomes unsolvable as the student has to assume the reaction.
The question should be awarded as a bonus
____________________________________________________________________________
Apart from the above-mentioned questions, there were questions in the integer section which had quite lengthy calculations, an issue which Careers360 has highlighted before. In some of these questions, it was impossible for students to arrive at the integer answer given by NTA in the answer keys without using a calculator.
Subscribe to Membership Plan
*Unlock all premium content and benefits:
Rate this Story
Latest Stories
MBBS In Russia? What You Must Know About Medical Colleges In Russia For Indian Students

Indian graduates who have completed MBBS from Russia cleared the FMGE exam and the success rate was 25%. Check here the Complete detail in this article.
By
7 min read ⋆ 16 Apr'24
MBBS In Russia: 25% Indian Graduates Cleared FMGE in 2022
7 min read ⋆ 16 Apr'24
Know the JEE Main 2023 cutoffs for NITs, IIITs and GFTIS
9 min read ⋆ 14 Apr'24
List of NITs Accepting Rank Below 2 Lakh in JEE Main
4 min read ⋆ 8 Apr'24
Careers360 helping shape your Career for a better tomorrow
250M+
Students
30,000+
Colleges
500+
Exams
1500+
E-Books
