How Many Allotropes of Carbon
Diamond, graphite, and fullerene—on top of others—all have very special characteristics and usages and are the allotropes of carbon. The versatility of using diverse carbon allotropes has revolutionized industries that are as diverse as jewelers and technologists.
This Story also Contains
- What are Carbon Allotropes?
- Some Solved Examples
- Summary
What are Carbon Allotropes?
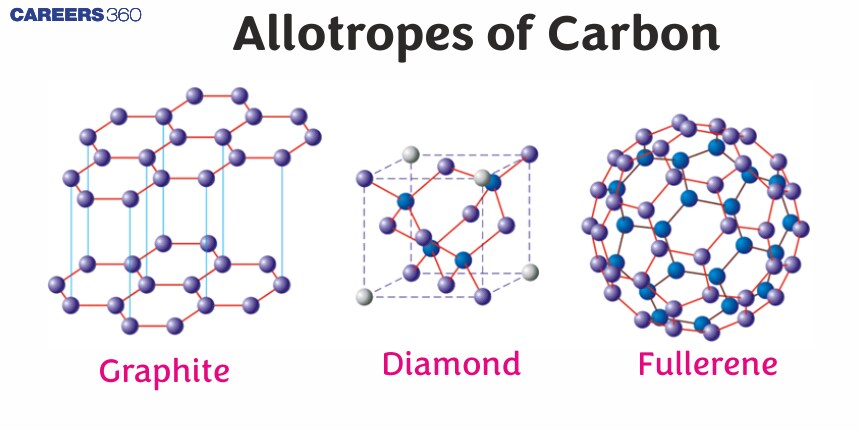
The allotropes are formed by the same atomic element, although atoms are held together by different bonds. Now, an element as versatile as this should be found in many different allotropes, mostly in diamond, graphite, and fullerenes. In contrast, each carbon atom in a diamond forms during the creation of four strong covalent bonds a tetrahedral arrangement that gives a great deal of hardness to the mineral. On the other hand, graphite shows a planar hexagonal lattice where each carbon atom is bonded to three others; thus, layers of atoms embody a natural mechanical lubrication, which makes it soft and slippery. Another type of carbon molecule, the fullerenes, has atoms arranged in a hollow sphere, ellipsoid, or tube. Again, the structural differences are a result of the bonding arrangement of the carbon atoms. The variety of structures realized in various carbon allotropes is truly mind-boggling.
Types of Carbon Allotropes
Carbon has mainly three allotropes: diamond, graphite, and fullerenes.
Diamond: Due to its incomparable hardness and high refractive index, it finds enormous applications in cutting tools and Jewelry. The bonding structure has a perfect tetrahedral lattice that makes this mineral the hardest known natural material.
Graphite, having been turned into this soft and lubricative form, has overwhelming usage in such important devices as pencils, lubricants, and batteries. The layer structure may be allowed to glide over each other that way, hence leaving its mark on paper.
Fullerenes: This includes buckminsterfullerene—a molecule resembling a soccer ball—and carbon nanotubes with unique electrical properties but with large tensile strength. Fullerenes are regarded to be of immense interest in the conduits of nanotechnology and material science due to their high tensile strength and electrical conductance.
The properties among the different allotropes vary because of the difference in the structures made by the carbon atoms; hence, it is an element of huge versatility and value in many applications.
Relevance and Applications
These carbon allotropes have found applications both in industries and in academics on a very large scale.
Diamond
Other than use in jewelry, where the application has strong marketing in the fashion world, the hardness attribute of the diamond recommends it for use in cutting, grinding, and drilling tools. Moreover, its high thermal conductance finds application in heat sinks, for instance, laser diodes and high-power transistors.
It has a crystalline lattice. In diamond, each carbon atom undergoes sp3 hybridization and is linked to four other carbon atoms by using hybridized orbitals in a tetrahedral fashion. The C–C bond length is 154 pm. The structure extends in space and produces a rigid three-dimensional network of carbon atoms. In this figure given below directional covalent bonds are present throughout the lattice. It is very difficult to break extended covalent bonding and, therefore, diamond is the hardest substance on the earth. It is used as an abrasive for sharpening hard tools, in making dyes, and in the manufacture of tungsten filaments for electric light bulbs.

Graphite
Graphite has a layered structure. Layers are held by van der Waals forces and the distance between two layers is 340 pm. Each layer is composed of planar hexagonal rings of carbon atoms. C—C bond length within the layer is 141.5 pm. Each carbon atom in a hexagonal ring undergoes sp2 hybridization and makes three sigma bonds with three neighboring carbon atoms. The fourth electron forms a π bond. The electrons are delocalized over the whole sheet. Electrons are mobile and, therefore, graphite conducts electricity along the sheet. Graphite cleaves easily between the layers and, therefore, it is very soft and slippery. For this reason, graphite is used as a dry lubricant in machines running at high temperatures, where oil cannot be used as a lubricant.

Fullerenes
Fullerenes are made by the heating of graphite in an electric arc in the presence of inert gases such as helium or argon. The sooty material formed by condensation of vapourised Cn small molecules consists of mainly C60 with a smaller quantity of C70 and traces of fullerenes consisting of an even number of carbon atoms up to 350 or above. Fullerenes are the only pure form of carbon because they have smooth structures without having ‘dangling’ bonds. Fullerenes are cage-like molecules. C60 molecule has a shape like a soccer ball and is called Buckminsterfullerene.
It contains twenty-six-membered rings and twelve five-membered rings. A six-membered ring is fused with six or five-membered rings but a five-membered ring can only fuse with six-membered rings. All the carbon atoms are equal and they undergo sp2 hybridisation. Each carbon atom forms three sigma bonds with other three carbon atoms. The remaining electron at each carbon is delocalized in molecular orbitals, which in turn give aromatic character to the molecule. This ball-shaped molecule has 60 vertices and each one is occupied by one carbon atom it also contains both single and double bonds with C–C distances of 143.5 pm and 138.3 pm respectively. Spherical fullerenes are also called buckyballs in short.

It opens an entrance into the world of molecular structure, bonding, and material science in relation to academics. Research into carbon nanotubes and graphene is expected to open the horizon wide on what is achievable in technology and engineering with materials, as they are also expected to hold out many potential innovations and applications further not far into the future.
Recommended topic video on (Allotropes of Carbon )
Some Solved Examples
Example 1
Question:
In graphite and diamond, the percentage of p-characters of the hybrid orbitals in hybridization are respectively:
1) 33 and 25
2) 33 and 75
3) 50 and 75
4) 67 and 75
Solution
As we have learned,
Graphite has $\mathrm{sp}^2$ hybridization
$\% \mathrm{p}-$ character $=\frac{2}{3} \times 100=67 \%$
Diamond has $\mathrm{sp}^3$ hybridisation
$\% \mathrm{p}-$ character $=\frac{3}{4} \times 100=75 \%$
Hence, the answer is the option (4).
Example 2
Question:
The tungsten filament for electric bulbs is formed by:
1) Graphite
2) Diamond
3) Fullerene
4) Charcoal
Solution:
Diamond is used for sharpening hard tools, making dies, jewellery, and tungsten filaments for electric bulbs.
Hence, the answer is option (2).
Example 3
Question:
The (mathrm{C - C}) bond length is maximum in:
1) Graphite
2) (${C_{70}}$)
3) (${C_{60}}$)
4) Diamond
Solution:
Graphite and Fullerenes(${C_{70}}$) and(${C_{60}}$) have a partial double bond character between the carbon atoms due to conjugation. Diamonds, on the other hand, contain only singly bonded carbon atoms, resulting in the greatest bond length.
Hence, the answer is option (4).
Summary
The several different carbon allotropes amply show the wonders of carbon versatility. It is with respect that the hardness of diamonds, the lubricating power of graphite, and the innumerable applications of fullerenes all demonstrate diverseness in the manner carbon atoms can bond and orient themselves. To be able to comprehend the allotropes we enrich our minds in chemistry but driving innovations in technology and industry. Inevitably, as carbon has a very deep root in our lives and science, with the advancement of research, the list of their potential applications grows.
