Galvanic Cells
The galvanic cell, also known as the Voltaic pile, consists of alternating layers of zinc and copper discs separated by pieces of paper soaked in saltwater. And it converts free energy into a chemical cell into an electrical cell. Volta’s discovery was driven by his interest in understanding the nature of electricity. Volta's galvanic cell was the first device capable of providing a steady electrical current.
This Story also Contains
- Galvanic Cells
- Some Solved Examples
- Summary
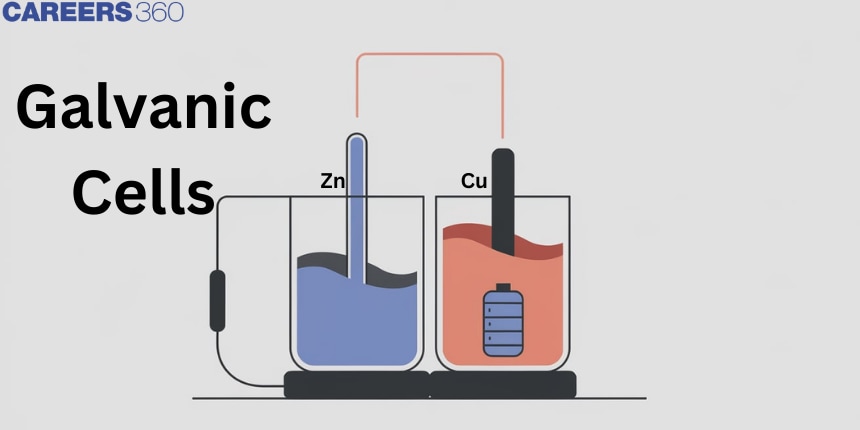
Galvanic Cells
It is the device in which the decrease of free energy during the indirect redox reaction is made to convert chemical energy into electrical energy. Luigi Galvani and Alessandro Volta developed such devices therefore these cells are also known as Galvanic cells Voltaic cells or Redox cells.
Species having a greater tendency to get oxidized (greater oxidation potential value ) is selected as the anode while species having a greater tendency of getting reduced (greater reduction potential value) is selected as a cathode.
| Feature | Cathode | Anode |
| Sign | Positive due to consumption of electrons | Negative due |
| Reaction | Reduction | Oxidation |
| Movement of electrons | Into the cell | Out of cell |
Galvanic Cell

- The Daniel cell is a typical galvanic cell. It is designed to make use of the spontaneous redox reaction between zinc and cupric ions to produce an electric current.
- The Daniel cell can be conventionally represented as $\underset{\text { Saltbridge }}{\mathrm{Zn}(\mathrm{s})\left|\mathrm{ZnSO}_4(\mathrm{aq})\right|\left|\mathrm{CuSO}_4(\mathrm{aq})\right| \mathrm{Cu}(\mathrm{s})}$ Zn(s)|ZnSO4(aq)||CuSO4(aq)|Cu(s) Saltbridge
- The Daniel cell reaction is represented as: $\mathrm{Zn}(\mathrm{s})+\mathrm{Cu}^{2+}(\mathrm{aq}) \rightarrow \mathrm{Zn}^{2+}(\mathrm{aq})+\mathrm{Cu}(\mathrm{s})$ Zn(s)+Cu2+(aq)→Zn2+(aq)+Cu(s)
- In Daniel's cell, electrons flow from the zinc electrode to the copper electrode through the external circuit while metal ions flow from one-half cell to the other through a salt bridge.
- Here current flows from the copper electrode to the zinc electrode that is, cathode to anode in an external circuit.
- Daniel cell is a reversible cell while a voltaic cell may be reversible or irreversible depending upon the e.g. if one of the products is gaseous and escapes, then the cell is not reversible.
| Electrochemical Cell | Electrolytic Cell |
It is a combination of two half cells, containing the same or different electrodes in the same or different electrolytes. | It is a single cell containing the same electrodes present in the same electrolyte. |
The anode is negative, the cathode is positive | The anode is positive, the cathode is negative |
Electrons move from anode to cathode in the external circuit. | Electrons enter through cathode and leave through the anode. |
It converts chemical energy into electrical energy, produced as a result of a redox reaction. | It converts electrical energy into chemical energy. Energy is supplied to the electrolytic solution to bring about the redox reaction. |
The cell reaction is spontaneous. | Cell reaction is non-spontaneous. |
Salt bridge is required. | No salt bridge is required. |
Recommended topic video on(Galvanic Cells)
Some Solved Examples
Example.1
1. Which of the following reactions is possible at the anode?
1) (correct)$2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}$
2)$\mathrm{F}_2 \rightarrow 2 \mathrm{~F}^{-}$
3)$\frac{1}{2} \mathrm{O}_2+2 \mathrm{H}^{+} \rightarrow \mathrm{H}_2 \mathrm{O}$
4)none of these
Solution
Let's look at the oxidation states of the reactants and the products both
$2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O} \rightarrow \stackrel{6}{\mathrm{Cr}_2} \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}$
Here Cr goes from +3 OS to +6 OS, this oxidation reaction is possible at the anode.
$\stackrel{0}{F}_2 \rightarrow 2 \mathrm{~F}^{-}$
This is a reduction reaction where the O.S of F changes from 0 to -1. Reduction reactions are not possible in an anode.
$\frac{1}{2} \mathrm{O}_2+2 \mathrm{H}^{+} \rightarrow \mathrm{H}_2 \mathrm{O}$
This is also a reduction reaction where the OS of oxygen changes from 0 to -2. Reduction reactions are not possible at the anode.
Hence, the answer is the option (1).
Example.2
2. Two half cells have potential -0.36 and 0.82 Volts respectively. These two are coupled to make a galvanic cell. Which of the following will be true?
1) (correct)The electrode of half cell potential -0.36 V will act as an anode
2)The electrode of half cell potential -0.36 V will act as a cathode
3)The electrode of half-cell potential 0.82 V will act as an anode
4)The electrode of half-cell potential -0.36 V will act as the positive terminal
Solution
Electrode potentials are conventionally Reduction potentials which denote the ability of a species to get reduced. The greater the reduction potential, the greater the ability to get reduced.
Correspondingly, an electrode with a negative reduction potential has a greater tendency to get oxidized and would act as an anode.
Since the electrode with potential -0.36 is more negative than the electrode with potential 0.82, therefore it will act as an anode and oxidation will occur there.
Hence, the answer is the option (1).
Example.3 Which of the following statements is correct about the Galvanic cell?
1) (correct)The anode is negatively charged
2)The cathode is negatively charged
3)Oxidation occurs at the cathode
4)Reduction occurs at the anode
Solution
The anode is negatively charged due to the release of electrons. Oxidation occurs at the anode always.
Hence, the answer is the option (1).
Example.4
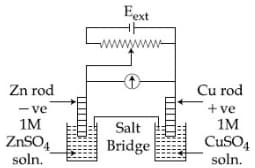
4.
![]()
$\mathrm{E}_{\mathrm{Cu}^2+\mid \mathrm{Cu}}^0=+0.34 \mathrm{~V}$
$\mathrm{E}_{\mathrm{Zn}^2+\mid \mathrm{Zn}}^o=-0.76 \mathrm{~V}$
Identify the incorrect statement from the options below for the above cell:
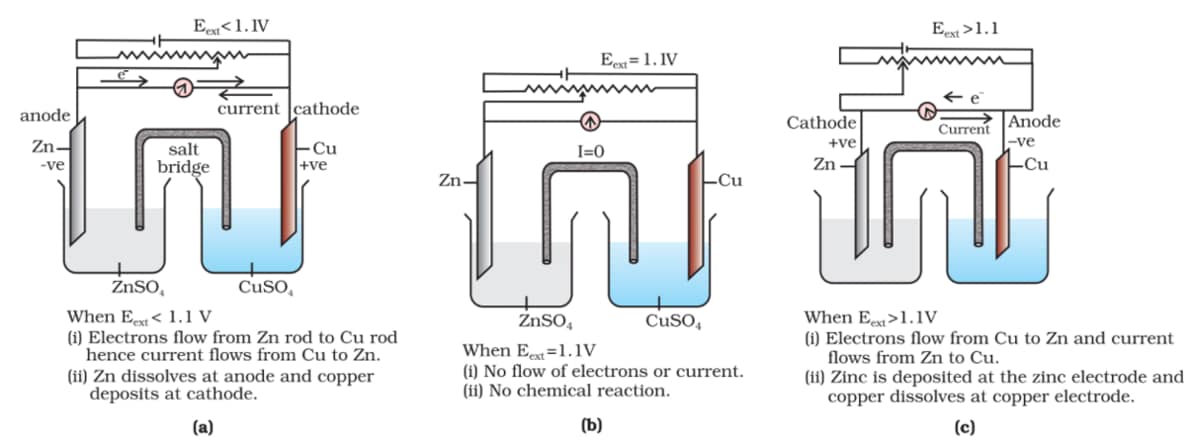
1)If Eext > 1.1 V, e- flows from Cu to Zn
2) (correct)If Eext > 1.1 V, Zn dissolves at Zn electrode, and Cu deposits at the Cu electrode
3)If Eext < 1.1 V Zn dissolves at the anode and Cu deposits at the cathode
4)If Eext = 1.1 V, no flow of e- or current occurs
Solution
We know,
$\begin{aligned} & \mathrm{E}_{\text {cell }}^o=\mathrm{E}_{\text {right }}^{\mathrm{o}}-\mathrm{E}_{\text {left }}^o \\ & \mathrm{E}_{\text {cell }}^{\mathrm{o}}=0.34-(-0.76) \\ & \mathrm{E}_{\text {cell }}^o=1.10 \mathrm{volt}\end{aligned}$
![]()
Incorrect statement - If Eext > 1.1 V, Zn dissolves at Zn electrode, and Cu deposits at the Cu electrode
Therefore, the correct option is (2).
Example.5 Which of the following can be classified as a chemical cell?
1)Electrolytic cell
2)Galvanic cell
3)Daniel Cell
4) (correct)2 & 3
Solution
Chemical Cells - The cells in which electrical energy is produced from the energy change accompanying a chemical reaction or a physical process are known as chemical cells.
The Electrolytic cell is a device in which electrolysis is carried out by using electricity or in which the conversion of electrical energy into chemical energy is done.
The Galvanic cell is a device in which the redox reactions lead to the conversion of chemical energy into electrical energy.
A Daniell cell is a galvanic cell that converts chemical energy into electrical energy by using the spontaneous redox reaction between zinc and cupric ions.
Hence, the answer is the option (4).
Summary
Alessandro Volta's work was so influential that the unit of electric potential, the volt, was named in his honor. His invention revolutionized the way electricity was understood and utilized, paving the way for future innovations in science and technology. Galvaniv call has various significance in such a way that the Electrochemical Principle as Volta’s cell was the first to provide a stable and continuous electrical current.
