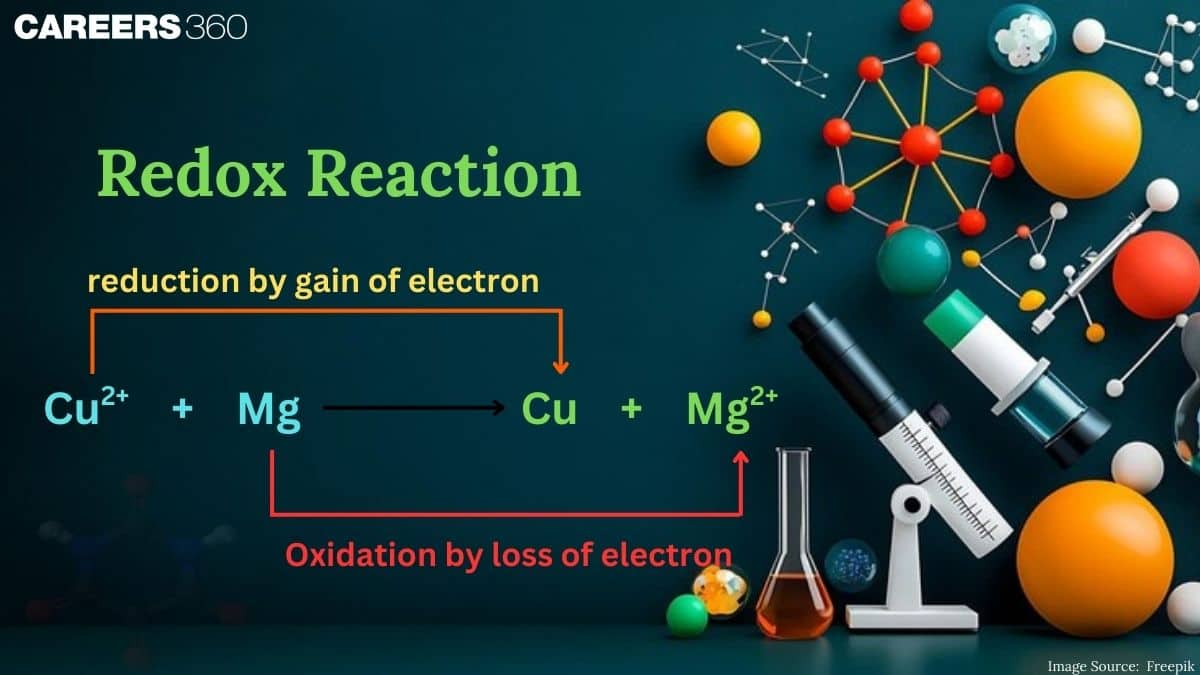
Redox Reaction
What happens when iron rusts while copper remains shiny? How does a battery generate electricity using chemicals?The answer to these question lies in this chapter Redox Reaction. You will in this chapter how the reaction like combustion, respiration, corrosion, and photosynthesis occur. A redox reaction is a chemical reaction in which both oxidation and reduction occur simultaneously. In a redox reaction, the substance that loses electrons is oxidized, and the substance that gains electrons is reduced.
This Story also Contains
- Important Topics of Redox Reaction
- How To Prepare For Redox Reaction?
- Redox Reactions in Different Exams
- Study Links for Redox Reactions
- Subject Wise Resources of NCERT
- Previous Year Question Of Redox Reaction
- Prescribed Books
- Conclusion
Chemistry explores how matter transforms, and one key process is redox reactions, where oxidation and reduction occur together. These reactions are essential in metal extraction like reducing iron ore or in electroplating to coat items with metals like gold.They power batteries and fuel cells, converting chemical energy to electricity, and are used in industrial electrolysis to produce chlorine or aluminum. Redox processes also clean water, create bleach, and drive biological functions such as respiration and photosynthesis. Across industries from pharmaceuticals to agricaulture they form the backbone of critical technologies.
Important Topics of Redox Reaction
Redox reactions form a fundamental part of chemistry, explaining how electrons are transferred between substances. These concepts of Chemistry help in understanding processes such as corrosion, electrochemistry, and energy production.
Redox Reaction
A redox reaction is a chemical reaction in which both reduction (gain of electrons) and oxidation (loss of electrons) occur simultaneously. It plays an important role in various chemical and biological processes, including respiration, photosynthesis, and industrial reactions like metal extraction and corrosion prevention. Redox reactions are often identified by changes in oxidation states of elements involved.
Oxidation Number
The oxidation number represents the hypothetical charge an atom would have if all bonds were ionic. It is used to track electron transfers in redox reactions. Rules for determining oxidation number includes assigning a value of 0 to free elements, matching the charge in ions, and following the standard rules for compounds and polyatomic ions. This concept helps balance redox equations systematically.
The oxidation number (ON) is a concept used to track the transfer of electrons in redox reactions. It is assigned to elements in compounds based on a set of rules:
- Free elements have an ON of 0.
- For monoatomic ions, the ON equals the ion’s charge.
- Oxygen typically has an ON of -2, except in peroxides (-1) and superoxides (-1/2).
- Hydrogen is +1 when bonded to non-metals and -1 when bonded to metals.
The concept is vital for identifying oxidized and reduced species in complex reactions and for balancing equations.
Displacement Reaction
In a displacement reaction, a more reactive element displaces a less reactive element from its compound. These reactions are classified into two types: metal displacement (e.g., zinc displacing copper from copper sulfate) and non-metal displacement (e.g., chlorine displacing bromine in bromide salts). Displacement reactions are a practical application of redox reactions, highlighting the reactivity series of elements.
Displacement reactions are a type of redox reaction where a more reactive element displaces a less reactive one from its compound. They are classified into two types:
1. Metal Displacement: A metal in its elemental form displaces another metal ion in a compound e.g
$$\mathrm{Zn}+\mathrm{CuSO}_4 \rightarrow \mathrm{ZnSO}_4+\mathrm{Cu}$$
2. Non-metal Displacement: A more electronegative non-metal displaces a less electronegative one e.g.,
$$\mathrm{Cl}_2+2 \mathrm{KI} \rightarrow 2 \mathrm{KCl}+\mathrm{I}_2$$
3. These reactions are widely used in metallurgy, electrochemical cells, and various industrial processes.
Balancing Redox Reactions
Balancing redox reactions ensures the conservation of mass and charge. The two primary methods are:
- Oxidation Number Method: The changes in oxidation numbers are used to balance the equation.
- Ion-Electron (Half-Reaction) Method: The oxidation and reduction half-reactions are balanced separately and combined.
These methods are essential for accurately representing chemical processes in aqueous and non-aqueous systems.
Types of Redox Reactions
Redox reactions can be broadly classified into the following types:
Combination Reactions: Two or more reactants combine to form a single product. In many cases, one reactant is oxidized while the other is reduced.
Example: $2 \mathrm{H}_2+\mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}$
Decomposition Reactions: A single compound breaks down into two or more simpler substances. Often, one product is oxidized while the other is reduced. Example: 2H2O → 2H2 + O2
Disproportionation Reactions: A single substance undergoes both oxidation and reduction simultaneously.
Example: $2 \mathrm{H}_2 \mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_2$
Standard Electrode Potential $\left(E^{\circ}\right)$
The standard electrode potential is a measure of the tendency of a chemical species to get reduced. It is measured in volts (V) under standard conditions (298 K, 1 M concentration, and 1 atm pressure).
- A positive E° indicates a strong tendency to be reduced, acting as a good oxidizing agent.
- A negative E° implies a strong tendency to be oxidized, acting as a good reducing agent.
Redox Reactions in Everyday Life
Redox reactions play an important role in various natural and industrial processes, such as:
- Corrosion: The oxidation of metals, especially iron (rusting), is a detrimental redox reaction requiring mitigation.
- Combustion: The burning of fuels (e.g., $\mathrm{CH}_4+2 \mathrm{O}_2 \rightarrow \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}$) involves redox reactions to release energy.
Electrochemical Applications of Redox Reactions
Redox reactions are fundamental to the working of electrochemical cells:
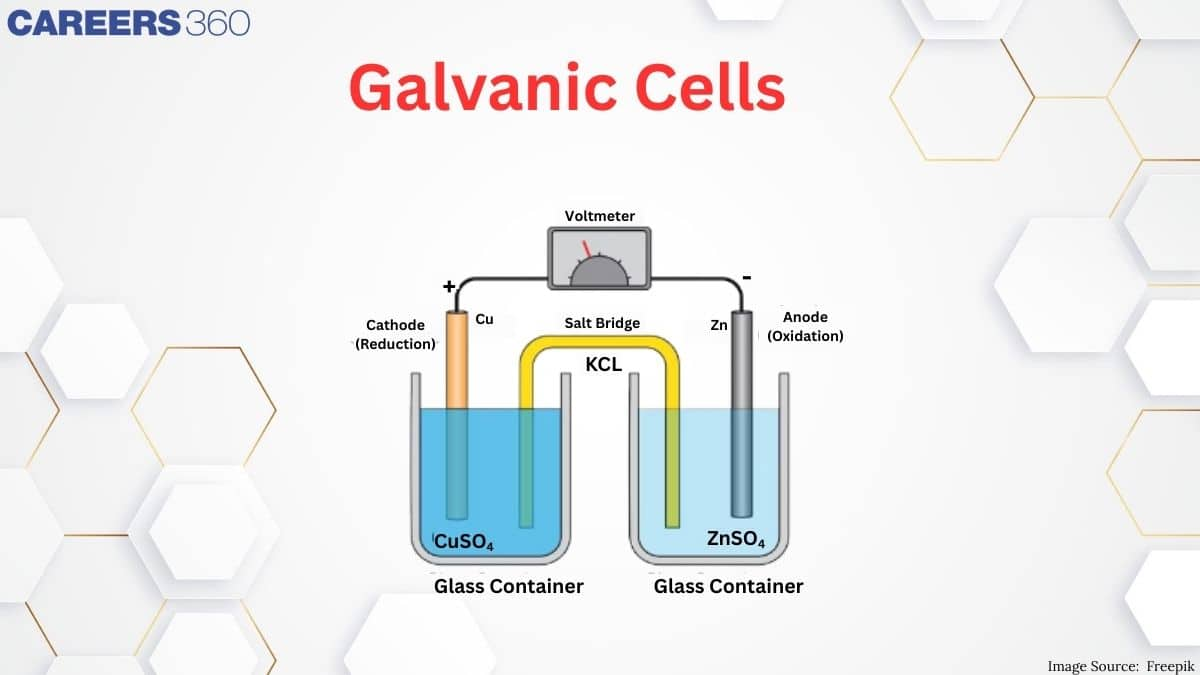
1. Galvanic Cells: Convert chemical energy into electrical energy using spontaneous redox reactions.
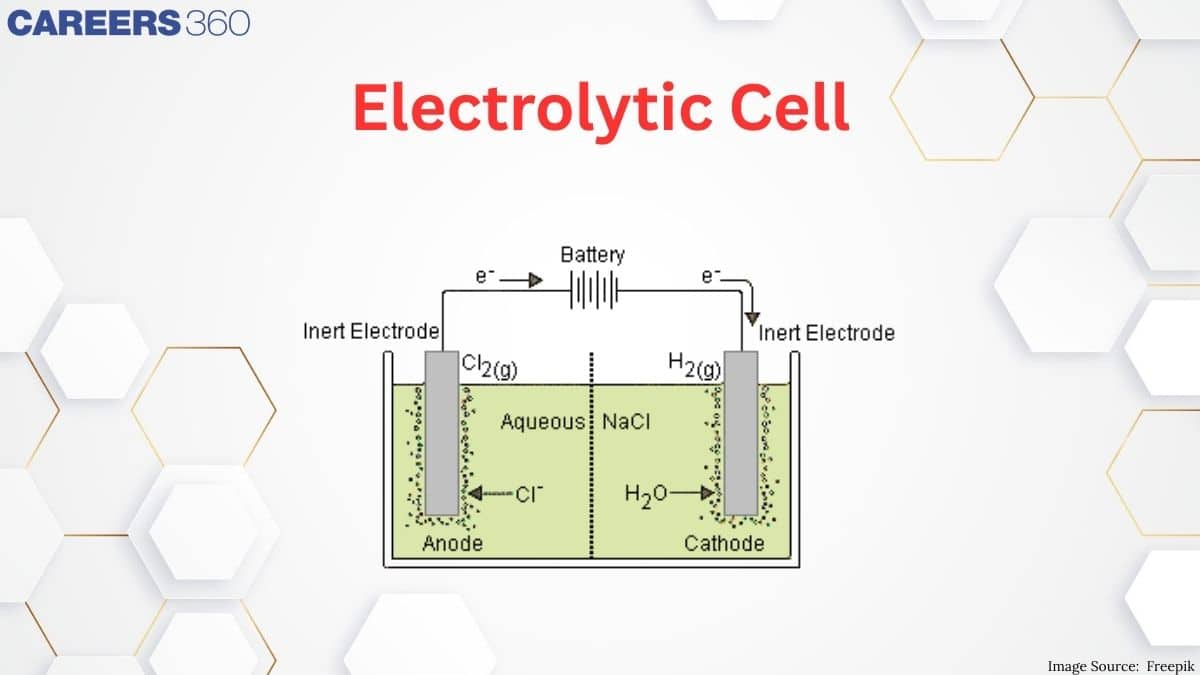
2. Electrolytic Cells: Use electrical energy to drive non-spontaneous redox reactions, as seen in electrolysis processes.
3. Batteries: Function on redox principles, with the flow of electrons from the reducing agent to the oxidizing agent generating electricity.
How To Prepare For Redox Reaction?
This physical‑chemistry chapter teaches redox conceptually no formulas to memorize.
- Learn to assign oxidation numbers, split reactions into half‑reactions, balance atoms, charges, and electrons, then combine them.
- Practice regularly to build confidence. It Is based on understanding, not memorization perfect for clear, concept‑driven learning.
- Before reading this chapter, first, you must have the basic knowledge of Oxidation-reduction and associated concepts form Chapter: Some Basic Concept Of Chemistry
- Rest this complete chapter is very simple, just be regular and be consistent in your numerical practice.
Redox Reactions in Different Exams
This chapter is commonly asked in board and competitive examinations with focus on oxidation-reduction concepts, oxidation numbers, and balancing of redox equations.
| Exam Name | Focus Area | Common Topics Asked | Preperation Tips |
| CBSE Board | Conceptual understanding | Oxidation–reduction, balancing equations | Practise NCERT examples |
| JEE Main | Concept application | Oxidation number method, redox titrations | Solve numerical and MCQs |
| JEE Advanced | Analytical understanding | Complex redox balancing | Focus on stepwise reasoning |
| NEET | NCERT-based theory | Oxidation numbers, redox reactions | Revise NCERT thoroughly |
| State Board Exams | Theory-oriented | Definitions, redox concepts | Learn key terms |
| Chemistry Olympiads | Advanced application | Electrochemical redox processes | Practise high-level problems |
Study Links for Redox Reactions
These resources are designed to support learning in chemistry by providing clear explanations and practice material. They help students revise concepts, formulas, and applications of redox reactions effectively.
Subject Wise Resources of NCERT
This section provides organised study materials and useful links for each subject to support effective learning and exam preparation.
Previous Year Question Of Redox Reaction
These questions are an important part of chemistry exam preparation and help students understand the exam pattern. Practicing them improves conceptual clarity and problem-solving skills related to redox reactions.
Question 1: Which of the following oxidation reactions are carried out by both $\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7$ and $\mathrm{KMnO}_4$ in acidic medium?
A. $\mathrm{I}^{-} \rightarrow \mathrm{I}_2$
B. $\mathrm{S}^{2-} \rightarrow \mathrm{S}$
C. $\mathrm{Fe}^{2+} \rightarrow \mathrm{Fe}^{3+}$
D. $I^{-} \rightarrow \mathrm{IO}_3^{-}$
E. $\mathrm{S}_2 \mathrm{O}_3{ }^{2-} \rightarrow \mathrm{SO}_4{ }^{2-}$
Choose the correct answer from the options given below :
1) B, C and D only
2) A, D and E only
3) A, B and C only
4) C, D and E only
Answer:
$\mathrm{I}^{-} \stackrel{\mathrm{H}^{+}}{\rightarrow} \mathrm{I}_2$$\mathrm{I}^{-} \xrightarrow{\mathrm{OH}^{-}} \mathrm{IO}_3^{-}$$\mathrm{S}^{-2} \xrightarrow{\mathrm{H}^{+}} \mathrm{S}$$\mathrm{S}_2 \mathrm{O}_3^{2-} \xrightarrow{\mathrm{OH}^{-}} \mathrm{SO}_4^{2-}$$\mathrm{Fe}^{+2} \longrightarrow \mathrm{Fe}^{+3}$ $\mathrm{S}_2 \mathrm{O}_3^{2-} \xrightarrow{\mathrm{H}^{+}} \mathrm{S} \downarrow+\mathrm{SO}_4^{2-}$
Oxidation reactions are carried out by both $\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7$ and $\mathrm{KMnO}_4$ in acidic medium by: A, B and C
Hence, the correct answer is option (3).
Question 2: Match the List-I with List-II
|
List-I (Redox Reaction) |
List-II (Type of redox reaction) | ||
| A | $\mathrm{CH}_{4(\mathrm{~g})}+2 \mathrm{O}_{2(\mathrm{~g})} \quad \underset{2 \mathrm{H}_2 \mathrm{O}_{(\mathrm{f})}(\mathrm{g})}{\stackrel{\Delta}{\mathrm{CO}}}+$ | I |
Disproportionation reaction
|
| B | $\begin{aligned} & 2 \mathrm{NaH}_{(\mathrm{s})} \xrightarrow{\Delta} \\ & 2 \mathrm{Na}_{(\mathrm{s})}+\mathrm{H}_{2(\mathrm{~g})}\end{aligned}$ | II | Combustion reaction |
| C | $\begin{aligned} & \mathrm{V}_2 \mathrm{O}_{5(\mathrm{~s})}+5 \mathrm{Ca}_{(\mathrm{s})} \\ & \xrightarrow{\Delta} 2 \mathrm{~V}_{(\mathrm{s})}+ \\ & 5 \mathrm{CaO}_{(\mathrm{s})}\end{aligned}$ | III | Decomposition reaction |
| D | $2 \mathrm{H}_2+\mathrm{O}_2(\mathrm{aq}) \rightarrow \Delta 2 \mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{O}_2(\mathrm{~g})$ | IV | Displacement reaction |
Choose the correct answer from the options given below :
1) A-II, B-III, C-IV, D-I
2) A-II, B-III, C-I, D-IV
3) A-III, B-IV, C-I, D-II
4) A-IV, B-I, C-II, D-III
Answer:
(A) Combustion of hydrocarbon
(B) Decomposition into gaseous product.
(C) Displacement of ' V ' by ' Ca ' atom.
(D) Disproportionation of H2O2−1 into O−2 and O∘ oxidation states.
Hence, the correct answer is option (1).
Question 3: Which of the following electrodes will act as anodes, when connected to Standard Hydrogen Electrode?
(i) $A l / A l^{3+}, E^{\Theta}=-1.66$
(ii) $\mathrm{Fe} / \mathrm{Fe}^{2+}, E^{\Theta}=-0.44$
(iii) $\mathrm{Cu} / \mathrm{Cu}^{2+}, E^{\Theta}=+0.34$
$
(i v) F_2(g) / 2 F^{-}(a q), E^{\Theta}=+2.87
$
1) (ii) and (iii)
2) (i) and (ii)
3) (ii) and (iv)
4) (i) and (iv)
Answer:
It is the potential difference between the two terminals of the cell when no current is drawn from it. It is measured with the help of potentiometer or vacuum tube voltmeter.
Calculation of the EMF of the Cell
Mathematically, it may be expressed as
$\begin{aligned} & \mathrm{E}_{\text {cell }} \text { or } \mathrm{EMF}=\left[\mathrm{E}_{\text {red }}(\text { cathode })-\mathrm{E}_{\text {red }}(\text { anode })\right] \\ & \mathrm{E}_{\text {ell }}^{\circ} \text { or } \mathrm{EMF}^{\circ} \\ & =\left[\mathrm{E}_{\text {red }}^{\circ}(\text { cathode })-\mathrm{E}_{\text {red }}^{\circ}(\text { anode })\right]\end{aligned}$
- Characteristics of cell and cell potential.
- For cell reaction to occur the Ecell should be positive. This can happen only if Ered (cathode) > Ered(anode).
- Eo cell must be positive for a spontaneous reaction.
- It measures free energy change for maximum convertibility of heat into useful work.
- It causes the flow of current from the electrode of the higher Eo value to lower Eo value.
Difference between EMF and Cell Potential
| EMF | Cell Potential |
|
It is measured by potentiometer. |
It is measured by voltmeter. |
|
It is potential difference between two electrodes when no current is flowing in the circuit. |
It is potential difference between two electrodes when current is flowing through the circuit. |
|
It is maximum voltage obtained from cell. |
It is less than maximum voltage. |
|
It corresponds to the maximum useful work obtained from the galvanic cells. |
It does not correspond to maintain useful work obtained from Galvanic Cell |
The answer is the option (i) and (ii)
Explanation: They will act as anodes because both of the electrodes has a negative value of standard reduction potential.
Hence, the answer is the option (2).
Practice more questions from the link given below:
Prescribed Books
First, you must finish the class XI and XII NCERT textbook and solve each and every example and unsolved question given in it. Then for advanced level preparation like JEE and NEET, you must follow O.P. Tandon. You must definitely solve the previous year's papers. Meanwhile, in the preparation, you must continuously give the mock tests for the depth of knowledge. Our platform will help you with a variety of questions for deeper knowledge with the help of videos, articles and mock tests.
Conclusion
Redox reactions involve electron transfer one substance loses electrons, another gains. Mastering oxidation states and balancing half‑equations is vital for JEE Mains and NEET electrochemistry questions, titrations, and cell problems . Applications span batteries, fuel cells, metal extraction, electroplating, corrosion prevention, respiration, photosynthesis, water purification, and chemical manufacture . Understanding these concepts builds strong foundations for competitive exams and real‑world chemistry.
Frequently Asked Questions (FAQs)
Oxidation state is a conceptual tool to track electron movement and changes in reactivity. While the oxidation state of a monatomic ion is equal to its charge, for atoms within molecules or polyatomic ions, the oxidation state is assigned based on a set of rules and might not directly reflect the actual charge distribution.
Consider the reaction between sodium and chlorine: $2\text{Na(s)} + \text{Cl}_2\text{(g)} \rightarrow 2\text{NaCl(s)}$ Here, sodium (Na) loses an electron to become $\text{Na}^+$ (oxidation). Chlorine ($\text{Cl}_2$) gains electrons to become chloride ions ($\text{Cl}^-$) (reduction).
Because fluorine is a reactive element, it replaces chloride, bromide, and iodide ions in solution, and it reacts with water, displacing oxygen.
Reactions involving combinations (Combination reactions)
Reactions of decomposition (Decomposition reactions)
Reactions to displacement (Displacement reactions)
Reactions of disproportionation (Disproportionation reactions )
Because of the partial loss of electrons, hydrogen gets oxidised. The hydrogen atoms in water have less electron density near them than they had in the H 2 molecule, even though the loss is not complete enough to create ions. Because of a partial gain of electrons, the oxygen is decreased.
Copper oxide and magnesium react to form copper and magnesium oxide.
Originally, the term "oxidation" was used to describe reactions in which an element reacts with oxygen. Example: The oxidation of magnesium occurs in the reaction between magnesium metal and oxygen to generate magnesium oxide.
During oxidation, the oxidation number rises. When an element's oxidation number changes from 0 to 1, it is oxidised.
