Sulphur And Its Allotropic Forms: Properties, Structure and Example
Among the most "antique" primary substances known to humanity due to their weird yellow color and suffocating smell, sulphur was identified by people as something of enormous applicability surprisingly. You probably wouldn't believe how sulphur is very important in a great variety of uses in everyday life and industrial manufacture. Imagine a bright red-colored matchstick that sets a fire under the warm fireplace on a cold night, or fertilizers to ensure good crops to feed the increasing population of the planet; all this has been possible due to sulphur and its unique properties.
This Story also Contains
- Physical Properties of Sulphur - 1
- Physical Properties of Sulphur- 2
- Some Solved Examples
- Summary
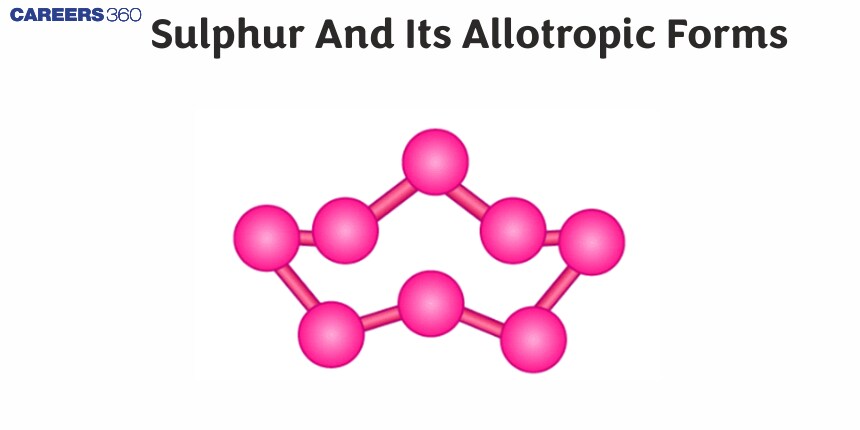
Physical Properties of Sulphur - 1
Sulphur is a nonmetallic element; it has an atomic number of 16. It has several allotropes which have a variety of different physical properties. Two of the major sulphur allotropes include rhombic and monoclinic sulphur as represented above. It appears rhombic in shape, is stable at room temperature, has a melting point of 115.21 °C, forms crystalline bright yellow colours, and is insoluble in water but soluble in carbon disulphide. Monoclinic monosulfur is stable at temperatures above 96 °C and up to the melting point of sulfur; the minerals exist in needle-like structures and are not soluble in water. Both allotropes are pale yellow in color and brittle in nature.
Physical Properties of Sulphur- 2
Besides rhombic and monoclinic sulfur, there are a couple of other allotropes in rare forms called plastic sulfur and amorphous sulfur.
If molten sulfur is cooled rapidly, then we get plastic sulfur which exhibits elasticity like rubber. Gradually bounce back to rhombic sulfur with the lapse of time. Amorphous sulphur, on the other hand, is a state where the sulphur has no crystalline structure and comes as a result of the very fast cooling of hot sulfur when it is dipped in cold water. It is dark in appearance and also rubbery, resembling the plastic sulphur, but it after some time changes back to crystalline sulphur. The physical properties are typical for the area of sulphur study and its application due to the uniqueness possessed by each of them. Sulphur and its allotropic forms have far-flung applications in varied fields.
For example, rhombic and monoclinic sulphur is vastly used in the production of sulphuric acid, one of the largest chemicals produced in industries. This acid is greatly needed in the production of fertilizers, during the refining of petroleum, and in the production of other chemicals and pharmaceuticals. About amorphous and plastic sulfur? How can this go to the rubber industry when it is known that they are both elastic and flexible? Also, it is used in vulcanization, a process where it increases the strength and elasticity of the articles that are made from rubber. Sulfur is also used in agriculture for the processing of fungicides and pesticides that are put to use to prevent disease and pests from attacking crops. Significant use in medicine is also attributed to the use of sulphurized compounds as skin condition products and as an agent against bacteria. The versatility of sulphur allotropes Rubin, 2001 underlines their significance to industry and the academic circle that they still become subject to more exploration and study.
Recommended topic video on (Sulphur And Its Allotropic Forms)
Some Solved Examples
Example 1
Question: Among the following allotropic forms of sulphur, the number of allotropic forms which will show paramagnetism is ______.
(A) (alpha)-sulphur
(B) (beta)-sulphur
(C)S2
Solution:
Hence, the answer is (1).
Example 2
Question: Which allotrope of sulphur is stable at room temperature?
1) Rhombic
2) Monoclinic
3) Plastic
4) Cyclo
Solution: Rhombic sulphur ((alpha)-sulphur) is the stable form at room temperature. It transforms to monoclinic sulphur ((beta)-sulphur) when heated above 369 K. Rhombic sulphur crystals are yellow, have a melting point of 385.8 K, and are insoluble in water but soluble in carbon disulfide.
Hence, the answer is (1) Rhombic.
Example 3
Question: Given below are two statements:
Statement-I:(alpha) and (beta) forms of sulphur can change reversibly between themselves with slow heating or slow cooling.
Statement-II: At room temperature, the stable crystalline form of sulphur is monoclinic sulphur.
In the light of the above statements, choose the correct answer from the options given below:
1) Statement I is false but Statement II is true
2) Both Statement I and Statement II are true
3) Statement I is true but Statement II is false
4) Both Statement I and Statement II are false
Solution: At room temperature, (alpha)-sulphur (rhombic sulphur) is the most stable form, not monoclinic sulphur. Therefore, Statement I is true but Statement II is false.
Hence, the correct answer is (3) Statement I is true but Statement II is false.
Summary
Sulphur is an element with a number of allotropic modifications which possess different physical properties and applications.
It has been reported that rhombic and monoclinic sulfur are the most occurring allotropes used for manufacturing in most industrial processes for sulfuric acid and rubber vulcanization. The special property forms are equivalent to plastic and amorphous sulfur, with applications set for them. Having elaborated on the physical properties of sulfur and different forms of sulfur and practical applications of sulfur, information has thus been made available on this very fundamental element. Admiration of the allotropes of this element and the practical consequences which are applied in our daily lives drives home the point on just how important the element is to most industries, among them agriculture, medicine, and manufacturing.
Frequently Asked Questions (FAQs)
The common allotropes of sulfur are rhombic, Ripple α-sulphur, and monoclinic, β-sulphur.
Rhombic sulfur is stable at room temperature, forming bright-yellow crystals, a melting point of 115.21°C. It is insoluble in water and soluble in carbon disulfide. Monoclinic sulfur is the form stable between 96°C and the melting point of sulfur. This form crystallizes in needle-like structures. Both structures are pale yellow and brittle. Knowing these allotropes is very vital for every utility of sulphur in different industries. 2. How is plastic sulphur formed?
Plastic sulphur, under rapid cooling of the molten sulphur, changes into a rubber-like, very elastic material.
S. Sulphur finds industrial application on a wide front.
Of all these, making sulfuric acid is arguably one example of the most important. Sulfuric acid is used in the making of fertilizers, in the refining of petroleum, and in making chemicals and pharmaceuticals. Another important application of Sulfur is in the vulcanizing of rubber to make the rubber material more tough and stretchable. It can also be used in fungicides and pesticides in agriculture production and aimed at protecting the crops from diseases and pests. This special flexibility makes sulphur of considerable value to a number of industrial processes.
Justifying the statement, it can easily be claimed that sulphur is vital in the production of fungicides and pesticide medicines in the sphere of Agriculture.
These sulphur-based compounds are used to treat diseases or pests in crops to result in higher yield and quality. On the second count, sulphur contributes toward improving the health of the soil; it promotes better uptake of nutrients and helps in the growth of plants. Application in agriculture is for sustainable maintenance of farming to cater for the global level of food supplies, and it has been an essential factor in recent times in modern agriculture.
Sulfur's allotropic nature permits it to exist in many physical forms that have a set of various unique properties that define it.
Sulphur has very vast applications in various industrial process.
The rhombic and monoclinic forms because it is quite stable and have very distinctive physical characteristics, it is used in the vulcanization of rubber and in making of sulfuric acid. The plastic and amorphous since it is very plastic and malleable it is used in searches for making rubber. Knowledge of the variation in allotropic forms of sulfur is openly required to exploit its heterogeneous properties at all places, right from industrial manufacturing to application in agriculture, and even for medicinal chemistry synthetic purposes.
