Tin (Stannum) Full Form
Tin is a chemical element. In the periodic table, this element belongs to the carbon family, group 14 (IV-a). This element is widely used for plating steel, iron, and other materials and their moulds like containers or cans, bearings, etc. The Latin name of tin is Stannum and it is represented by “Sn”. It is available in two forms in nature. They are organic tin and inorganic tin. Tin was discovered in the year around 3500 BC. It has isotopes and allotropes. At 20o C (68o F), the element tin is in a solid state. It has a tetragonal crystal structure.
This Story also Contains
- Characteristics of The Element Sn
- Properties of The Element Sn
- Isotopes And Allotropes of Tin
- Applications of Tin
- Compounds of Tin
Characteristics of The Element Sn
The characteristics of the element Tin includes its chemical formula, atomic mass, atomic weight, density, melting point, and boiling point, oxidations states, etc.
The chemical symbol for the element Tin is “Sn”.
Its electronic configuration is
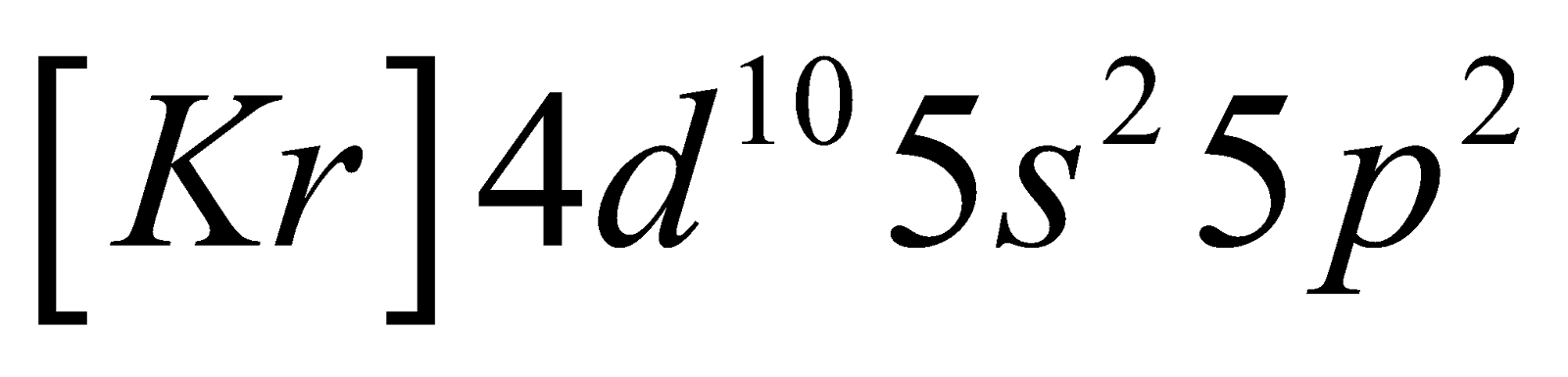 . $\left[ Kr \right]4{{d}^{10}}5{{s}^{2}}5{{p}^{2}}$
. $\left[ Kr \right]4{{d}^{10}}5{{s}^{2}}5{{p}^{2}}$Its atomic number and atomic mass are 50 and 118.71 U.
The oxidations states for the element Tin are +4 and +2.
This is a P-block element and a post-transition metal.
It has 8 stable isotopes.
There are three allotropes for Tin.
The boiling point of Sn is 2,270oC (4,100oF) and the melting point of Sn is 231.97oC (449.54oF).
Properties of The Element Sn
Tin is a soft silver-white metal with a bluish-white appearance.
It is a non-toxic, ductile, and malleable metal.
Its occurrence is rare comparatively.
Its concentration in the Earth’s crust is about 0.001%. But it is abundantly found with the alloy of other elements like cobalt, copper, nickel, etc.
It reacts with strong acids but doesn’t react with weaker acids.
This element exists in two forms or allotropes. They are white tin (beta tin) and grey tin (alpha tin).
Depending on the temperature, these forms change into one another.
Tin pest occurs at low temperatures and such metal is used in very cold regions.
In these two forms of tin, white tin has a body-centered tetragonal crystal structure and grey tin has a face-centered cubic structure.
Oxygen in the air shows an effect on Tin. But at room temperature, oxygen and water have no effect on Tin.
Since its low melting point and resistance to corrosion used as an oxidation-resistant coating material.
Isotopes And Allotropes of Tin
Tin has 8 stable isotopes that occur in different percentages.
There are two important allotropes of tin grey tin and white tin.
grey tin is also known as alpha tin.
The grey tin changes to white at 13.2oC (55.8oF).
The white tin is also known as beta tin.
The white tin changes to the rhombic crystal at higher temperatures above 100oC (212oF).
Applications of Tin
Applied as an oxidation-resistant coating material.
Used in plate glass production.
Applied in low-temperature casting alloys.
Used as a weighting agent for fabrics.
A compound like tin fluoride is used in dentifrices.
Used as a stabilizer in plastic and wood preservatives.
Used as an anti-fouling agent in boats and ships.
Applied in electrodes of batteries.
Widely used in food containers made of steel.
Compounds of Tin
Tin forms two types of compounds according to its oxidation state in the compound.
They are: Stannous (+2 state) and Stannic (+4 state)
Some examples of these compounds are: Stannous Chloride (SnCl2), Stannous oxide (SnO), Stannous Fluoride (SnF2), Stannic Chloride (SnCl4), Stannic Oxide (SnO2).
These compounds are used in many applications such as for galvanizing, chemical reagents, plating, toothpaste, perfumes, and as a catalyst in industrial applications.
Frequently Asked Questions (FAQs)
No, Sn occurs rarely. Its extraction from the Earth’s crust is about 0.001%.
The element Sn belongs to the carbon family, group 14 (IV-a) in the periodic table.
Humans directly do not consume tin. The tin is used as a coating for the steel cans in which food is stored. Thus, the interference of tin compounds with iron and copper decides its toxic effect.
The atomic number of the tin is 50.
The chemical name of the element represented by Sn is “Tin”.