Reverse Osmosis: Definition, Process, Uses, Working of Reverse Osmosis
Reverse osmosis is a water purification process that uses pressure to force water through a semipermeable membrane against its concentration gradient. It removes dissolved salts and impurities efficiently. RO is vital in desalination, filtration, and biological systems. The working principle or the mechanism, advantages, and some limitations of reverse osmosis are overviewed here to understand this important technology in some detail.
This Story also Contains
- What is Reverse Osmosis?
- Principle of Reverse Osmosis
- Process of Reverse Osmosis
- Reverse Osmosis Experiment
- Advantages of Reverse Osmosis
- Disadvantages of Reverse Osmosis
- Reverse Osmosis NEET MCQs (With Answers & Explanations)
- Recommended Video on Reverse Osmosis

What is Reverse Osmosis?
Reverse osmosis (RO) is a separation technique majorly applied in water purification. RO primarily involves forcing water through a semipermeable membrane to remove dissolved solutes. It is particularly very effective in desalination of seawater and purification of drinking water.
This process became more effective with the development of membrane technology and polymers, thus turning it into one of the important tools for residential and industrial applications.
Principle of Reverse Osmosis
The reverse osmosis process relies on a semipermeable membrane, where significantly only the solvent molecules are allowed to pass through while blocking larger solute molecules. During the process, the pressure applied to the solution side of the membrane ensures that the solvent molecules move through to the side with a lower concentration of the solutes. This finally leads to the separation of solutes from the solvent, hence purification.
Process of Reverse Osmosis
The process of reverse osmosis has the following distinct steps associated with it:
Pressure Application: Pressure applied on the solution side is increased to a value above the osmotic pressure, which is the minimum pressure required to halt the flow of the solvent across the membrane.
Solvent Movement: Due to this elevated pressure, the solvent molecules move through the semipermeable membrane to the lower concentration side of the solute particles.
Separation and Purification: The solute particles get separated from the solvent, which becomes purified.
This process works effectively when pressure is applied that is greater than the osmotic pressure and on flow in the proper direction of the solvent.
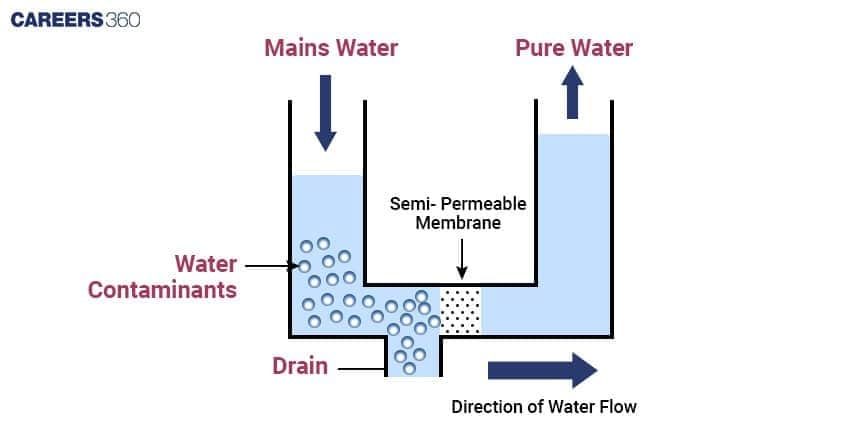
Reverse Osmosis Experiment
An illustrative experiment would do well to explain reverse osmosis:
Assembly: Take two containers, with a semipermeable membrane in between, one containing fresh water, and another containing a solution of high concentration.
Application of Pressure: Apply pressure to the concentrated solution side.
Result: Due to this, water molecules diffuse through the membrane into the freshwater side, thereby explaining the concept of reverse osmosis.
This simple experiment is a good example of how reverse osmosis works in a closed environment.
Advantages of Reverse Osmosis
Some of the advantages associated with reverse osmosis are enumerated below:
Decontamination: Efficiently removes different dissolved and suspended particles, including salts, chemicals, and microorganisms.
Wide applications: The technology ranges from desalination of seawater and industrial liquid wastes to drinking water purification.
Health benefits: This technology provides clean water and hence saves customers from waterborne diseases.
These advantages combine to make reverse osmosis an extremely useful technology both in the home and industrially.
Disadvantages of Reverse Osmosis
Despite all of the advantages, there are some reverse osmosis limitations:
Membrane Sensitivity: The various types of membranes used have varying tolerances to pH and temperature. For example, cellulose acetate membranes can not withstand high temperatures above 35°C, while polyamide membranes do not have good resistance to chlorine.
Maintenance Requirements: Membranes need periodic replacement. Sterilization of the system has to be done regularly to ensure efficiency.
Wastewater: In this process, a lot of wastewater is generated. This can create certain misgivings about the use of reverse osmosis in places where there are limited sources of water.
Reverse Osmosis NEET MCQs (With Answers & Explanations)
The key concepts to be covered under this topic for different exams are:
Principle of Reverse Osmosis
Advantages and disadvantages
Practice Questions for NEET
Q1. The osmotic expansion of a cell kept in water is chiefly regulated by:
Mitochondria
Vacuoles
Plastids
Ribosomes
Correct answer: 2)Vacuoles
Explanation:
The osmotic expansion of cells in water is primarily regulated by distinct structures across various organisms. In plant cells, fungi, and bacteria, the cell wall serves a crucial role in this process. This rigid structure provides the necessary support and resistance against excessive water intake via osmosis, thereby averting lysis. Additionally, the cell wall in plant cells helps maintain turgor pressure as the vacuole enlarges, thus preserving structural integrity.
Conversely, animal cells, devoid of a cell wall, rely on the plasma membrane's active ion transport mechanisms to manage osmotic balance. These cells are vulnerable to bursting in hypotonic surroundings without such regulatory systems.
Hence, the correct answer is option 2) Vacuoles.
Q2. What does osmotic pressure relate to?
The force of solutes in the solution
The force with which solvent is moving
The force with which solutes are moving
None of these
Correct answer: 2) The force with which solvent is moving
Explanation:
Osmotic pressure is a concept first introduced by Pfeffer and refers to the pressure applied on the solution side to prevent the movement of solvent particles towards the solution through a semipermeable membrane. It is equal and opposite to the force exerted by the solvent particles during osmosis. Osmotic pressure can also be defined as the excess hydrostatic pressure that must be applied to a solution to equalize its water potential with that of pure water. In simpler terms, it is the pressure required to stop osmosis. It is a measurable, positive force and a colligative property, meaning it depends on the number of solute particles in the solution, not their type. Osmotic pressure is often determined using an osmometer and is directly related to the movement of solvent particles toward the solution.
Hence, the correct answer is option 2) The force with which the solvent is moving.
Q3. Osmotic pressure is minimum in __________ and highest in ____________
Hydrophytes, Xerophytes
Xerophytes, Hydrophytes
Mesophytes, Xerophytes
Hydrophytes, Mesophytes
Correct answer: 1) Hydrophytes, Xerophytes
Explanation:
Osmotic pressure is least in hydrophytes due to their aquatic habitat, which naturally entails lower solute levels inside their cells.
Conversely, xerophytes exhibit maximum osmotic pressure, as they inhabit arid regions and must effectively maintain elevated solute concentrations within their cells to efficiently attract and hold water from the environment.
Hence, the correct answer is option 1) Hydrophytes, Xerophytes.
Also Read:
Recommended Video on Reverse Osmosis
Frequently Asked Questions (FAQs)
The maintenance includes periodic replacement of membranes, regular sterilisation of a system, and monitoring for any possible problems.
The amount of waste varies, but commonly, reverse osmosis systems can waste up to 3–4 times the amount of purified water produced.
Reverse osmosis is targeted at purifying water from dissolved salts, chemicals, and other impurities in the water.
Applied membranes are usually made of cellulose acetate, polyamide, and thin-film composites; each type has some features and applications.
