Acetone - Structure, Preparation, Uses, FAQs
Acetone solvent is also known as propanone and it is a type of organic compound that constitutes the formula (CH3)2CO. This compound is the simplest and smallest ketone known. Some of its property includes that it is colourless, highly volatile and flammable liquid and also constitutes a characteristic pungent smell.
Acetone is soluble in water and plays a role as an important organic solvent, also used in industries, also in home, and also in laboratories. Approx 6.7 million tonnes had been produced worldwide in the year 2010, and mainly used as a solvent and in the production of methyl methacrylate and bisphenol A. It is also a common building block in the field of organic chemistry. General household uses of acetone are that it is used as an active ingredient in nail polish remover and also as paint thinner. Whereas it also contains volatile organic compound (VOC).
This Story also Contains
- History of acetone?
- Method of preparation of acetone?
- Industrial use of acetone?
- What is acetone chemical ?
Acetone can be produced and disposed out from the human body through normal metabolic processes. It is generally present in blood and urine. People afftected with diabetic ketoacidosis produce it in very larger amounts. Reproductive toxicity tests proves that it has low potential to cause reproductive problems. Ketogenic diets that will increase ketone bodies throughout the blood are generally used to counter epileptic attacks in infants and also in the children who suffer from refractory epilepsy.
Also read -
History of acetone?
Acetone was first obtained by Andreas Libavius in the year 1606 through the process of distillation of Lead(II) acetate. In the year 1832, French chemist named as Jean-Baptiste Dumas and a German chemist named as Justus von Liebig gave the acetone the empirical formula. In the year 1833, there was a French chemist Antoine Bussy who named acetone. In acetone the suffix -one is added to the corresponding acid. By the year 1852, English chemist i.e. Alexander William Williamson realized that acetone is methyl acetyl and within the following year, the French chemist Charles Frédéric Gerhardt concurred that realization. The modern structural formula of acetone was published in year 1865 by a German chemist named as August Kekulé Johann Josef Schmidt had also presented the structure of acetone in the year 1861. Chaim Weizmann is the one who developed the process for industrial production of acetone and the process is known as Weizmann Process.
In the year 2010, the worldwide production ability for acetone was estimated around 6.7 million tonnes per year.
Acetone is generally produced directly or indirectly from propylene. About 83% of acetone is produced using the cumene process and as a result, acetone production is proportional to phenol production. In the cumene process itself , benzene is alkylated with propylene and then the product produce will be cumene, which is further oxidized by air to produce phenol and acetone.
Other processes that involves the direct oxidation of propylene are Wacker-Hoechst process and the hydration of propylene will be done to give 2-propanol, which is oxidized to form acetone.
C3H60 structure.
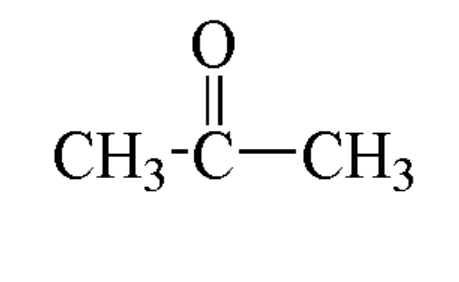
Method of preparation of acetone?
Previously, acetone can also be formed by dry distillation of acetates.
Example: ketonic decarboxylation of calcium
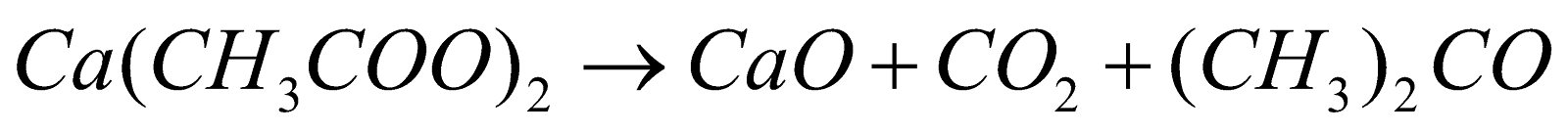
During World War I, acetone can be produced using acetone-butanol-ethanol fermentation with the help of Clostridium acetobutylicum i.e. a bacteria, and this method was developed by Chaim Weizmann, to help the British government in their war effort and this method is also used in the preparation of Cordite.
Acetone density
0.791 g/mL
About (CH3)2CO
It is a type of organic compound and constitute the formula (CH3)2CO. This compound is the simplest and smallest ketone known. Some of its property includes that it is colourless, highly volatile and flammable liquid and also constitutes a characteristic pungent smell.
Acetone is soluble in water and plays a role as an important organic solvent, also used in industries, also in home, and also in laboratories. Approx 6.7 million tonnes had been produced worldwide in the year 2010, and mainly used as a solvent and in the production of methyl methacrylate and bisphenol A. It is also a common building block in the field of organic chemistry. General household uses of acetone are that it is used as an active ingredient in nail polish remover and also as paint thinner. Whereas it also contains volatile organic compound (VOC).
Acetone can be produced and disposed out from the human body through normal metabolic processes. It is generally present in blood and urine. People afftected with diabetic ketoacidosis produce it in very larger amounts. Reproductive toxicity tests proves that it has low potential to cause reproductive problems. Ketogenic diets that will increase ketone bodies throughout the blood are generally used to counter epileptic attacks in infants and also in the children who suffer from refractory epilepsy.
Related Topics, |
Industrial use of acetone?
About one third of the world's acetone is used in industries as a solvent, and a quarter of acetone formed is consumed to form acetone cyanohydrin, which is a precursor of methyl methacrylate.
Solvent
Acetone is also a good solvent for plastics and many synthetic fibers. It is also used for thinning polyester resin, cleaning tools also uses acetone for their proper function, and dissolving two-part i.e. epoxies and superglue before they will become hard. It is also used as one of the volatile constituent in some paints and varnishes. Also as a heavy-duty degreaser a d it is also very useful in the preparation of metal that is prior to painting or soldering, and also it is used to remove rosin flux after soldering will be done (i.e. to prevent adhesion of dirt and electrical leakage ), although it can also attacks many of the electronic components ( such as polystyrene capacitors) so it is unfit for cleaning of circuit boards.
Also Read:
What is acetone chemical ?
Acetone that is also known as propanone and it is a type of organic compound and constitute the formula (CH3)2CO. This compound is the simplest and smallest ketone known. Some of its property includes that it is colourless, highly volatile and flammable liquid and also constitutes a characteristic pungent smell.
Acetone is soluble in water and plays a role as an important organic solvent, also used in industries, also in home, and also in laboratories. Approx 6.7 million tonnes had been produced worldwide in the year 2010, and mainly used as a solvent and in the production of methyl methacrylate and bisphenol A. It is also a common building block in the field of organic chemistry. General household uses of acetone are that it is used as an active ingredient in nail polish remover and also as paint thinner. Whereas it also contains volatile organic compound (VOC).
Acetone can be produced and disposed out from the human body through normal metabolic processes. It is generally present in blood and urine. People afftected with diabetic ketoacidosis produce it in very larger amounts. Reproductive toxicity tests proves that it has low potential to cause reproductive problems. Ketogenic diets that will increase ketone bodies throughout the blood are generally used to counter epileptic attacks in infants and also in the children who suffer from refractory epilepsy.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Questions related to
On Question asked by student community
Correct Answer: 1
Solution : The correct option is 1.
Acetone has the molecular formula C3H6O.
- Carbon (C) atoms: There are 3 carbon atoms.
- Hydrogen (H) atoms: There are 6 hydrogen atoms.
- Oxygen (O) atoms: There is 1 oxygen atom.
Therefore, in 1 molecule of
