Electronic Configuration of Iron - Definition, Structure, Application & Uses
Iron is a chemical element with atomic number 26 and symbol Fe, derived from the Latin word Ferrum. It is one of the most abundant transition metals found in the Earth's crust and plays a vital role in both industrial applications and biological systems. Iron belongs to the d-block (transition elements) and is well known for its ability to exhibit variable oxidation states, commonly +2 (ferrous) and +3 (ferric), though higher oxidation states such as +6 are also known in compounds like potassium ferrate $\left(\mathrm{K}_2 \mathrm{FeO}_4\right)$.
This Story also Contains
- Electronic Configuration of Iron (Fe)
- Calculate the Oxidation number for $\mathrm{FeSO}_4$
- Uses of Iron
- Some Facts About Iron
- Some Solved Examples
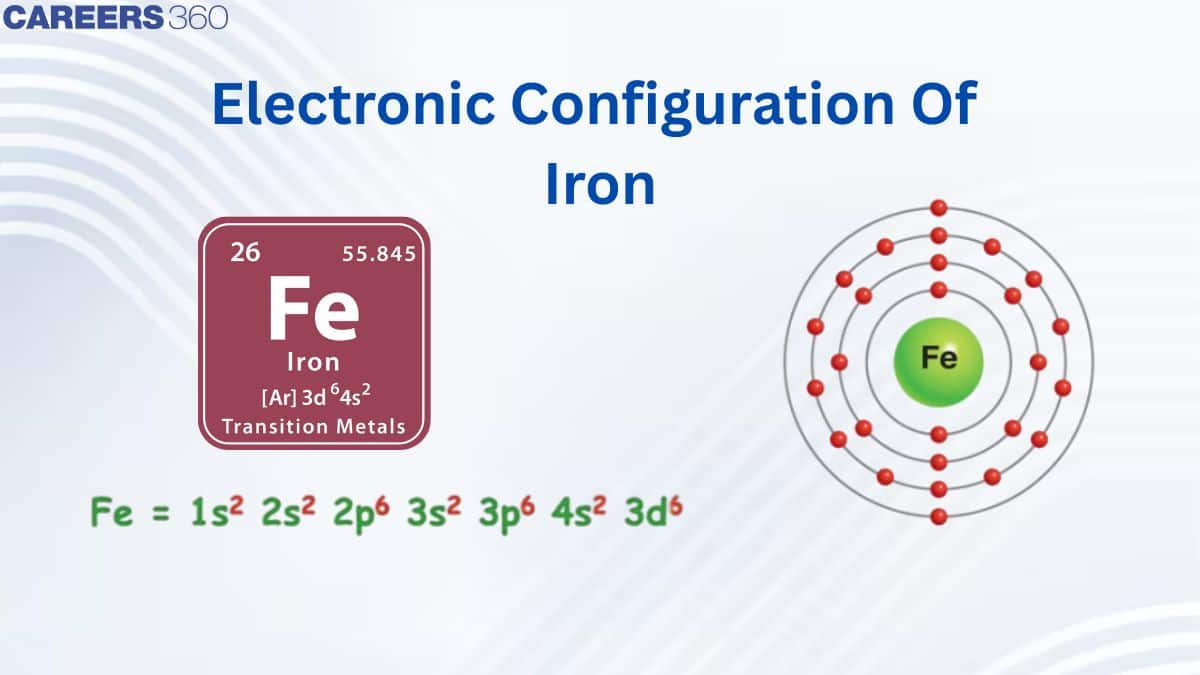
Electronic Configuration of Iron (Fe)
Iron (Fe) is a transition metal with atomic number 26, meaning it contains 26 electrons. Its electronic configuration in the ground state is $[\mathrm{Ar}] \mathrm{3d}^6 \mathrm{4s}^2$, where the first 18 electrons correspond to the noble gas argon, and the remaining eight electrons occupy the 4s and 3d orbitals. According to the Aufbau principle, the 4s orbital is filled before the 3d orbital, resulting in a complete electronic configuration of $1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 4 s^2 3 d^6$. The presence of partially filled 3d orbitals gives iron its characteristic transition metal properties, such as variable oxidation states, formation of complexes, and magnetic behavior. When iron forms ions, electrons are removed first from the 4s orbital; thus, the ferrous ion $\left(\mathrm{Fe}^{2+}\right)$ has the configuration $[\mathrm{Ar}] 3 \mathrm{~d}^6$, while the ferric ion $\left(\mathrm{Fe}^{3+}\right)$ has the more stable half-filled configuration $[\mathrm{Ar}] 3 \mathrm{~d}^5$.
Using Only the Periodic Table
-
Locate iron (Fe) in the periodic table.
-
Iron is in:
-
Period = 4
-
Group = 8
-
d-block (transition metal)
-
-
Fill electrons according to the position in the periodic table:
-
Up to Argon (Ar, Z = 18) → [Ar]
-
Remaining electrons = 26 − 18 = 8
-
-
In the 4th period:
-
4s orbital fills before 3d
-
Electrons fill as 4s², then 3d⁶
-
Electronic Configuration (Periodic Table Method):
$\mathrm{Fe}=[\mathrm{Ar}] 3 d^6 4 s^2$
Using the Electron Configuration Chart (Aufbau Principle)
According to the Aufbau principle, electrons fill orbitals in increasing order of energy:
Order of Filling:
$1 s \rightarrow 2 s \rightarrow 2 p \rightarrow 3 s \rightarrow 3 p \rightarrow 4 s \rightarrow 3 d$
| Orbital | Electrons |
|---|---|
| 1s | 2 |
| 2s | 2 |
| 2p | 6 |
| 3s | 2 |
| 3p | 6 |
| 4s | 2 |
| 3d | 6 |
Electronic Configuration (Aufbau Chart Method):
$1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 4 s^2 3 d^6$
Number of Electrons in Iron
Atomic mass - 55.845
Number of Protons - 26
Number of Neutrons- 30
Number of Electrons- 26
Related Topics link
Calculate the Oxidation number for $\mathrm{FeSO}_4$
The oxidation number of FeSO4 is calculated as 0, which is a neutral compound.
The first step is to identify the oxidation rate for each element present in the compound.
The oxidation number of S is 6.
The oxidation number of Fe is +2.
The oxidation number O is (-2 ✕ 4) = - 8
When the entire oxidation number of the conference is included, it provides
(+2) + (+6) + (-8) = 0
(+8) - 8 = 0.
Related Topics link
Uses of Iron
It is used to make metal and is also used in civil engineering such as reinforced concrete, girders etc.
Iron is used to make alloy metals such as carbon steel with additives such as nickel, chromium, vanadium, tungsten, and manganese.
These are used to make bridges, electric poles, bicycle chains, cutting tools, and gun barrels.
Iron ore contains 3-5% of carbon. It is used for making valves,pumps and pipes
Metal elements are used in Haber's process of producing ammonia.
Magnets can be made up of iron metal and its alloys and compounds.
Some Facts About Iron
1.The normal human body contains about 4 grams of iron in the form of hemoglobin, in the blood.
2.It is the sixth most common thing in the universe.
3.There are four known allotropic metals.
Also read -
Some Solved Examples
Question 1: The correct ground-state electronic configuration of iron ( $Z=26$ ) is:
A. $[\mathrm{Ar}] 3 \mathrm{~d}^8 4 \mathrm{~s}^0$
B. $[\mathrm{Ar}] 3 \mathrm{~d}^6 4 \mathrm{~s}^2$
C. $[\mathrm{Ar}] 3 \mathrm{~d}^7 4 \mathrm{~s}^1$
D. $[\mathrm{Ar}] 3 \mathrm{~d}^5 4 \mathrm{~s}^3$
Solution:
According to the Aufbau principle, electrons fill the 4 s orbital before the 3 d orbital. Iron has 26 electrons, so after $[\mathrm{Ar}]$ ( 18 electrons), the remaining 8 electrons fill as $4 s^2 3 d^6$.
$$
\mathrm{Fe}=[\mathrm{Ar}] 3 d^6 4 s^2
$$
Hence, the correct answer is option (C)
Question 2:The electronic configuration of the ferric ion $\left(\mathrm{Fe}^{3+}\right)$ is:
A. $[\mathrm{Ar}] 3 \mathrm{~d}^6$
B. $[\mathrm{Ar}] 3 \mathrm{~d}^4$
C. $[\mathrm{Ar}] 3 \mathrm{~d}^5$
D. $[\mathrm{Ar}] 4 \mathrm{~s}^1 3 \mathrm{~d}^4$
Solution:
Electrons are removed first from the 4s orbital.
$\begin{aligned}
& \mathrm{Fe} \rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^6 4 \mathrm{~s}^2 \\
& \mathrm{Fe}^{2+} \rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^6 \\
& \mathrm{Fe}^{3+} \rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^5
\end{aligned}$
Hence, the correct answer is option (C)
Question 3: Which of the following statements about iron is correct?
A. Iron has completely filled d-orbitals
B. Iron has half-filled d-orbitals in its ground state
C. Iron shows variable oxidation states due to partially filled d-orbitals
D. Iron does not form complexes
Solution:
Iron has partially filled 3d orbitals $\left(3 d^6\right)$, which allow it to lose different numbers of electrons and form complexes, resulting in variable oxidation states.
Hence, the correct answer is option (C)
Question 4: The number of unpaired electrons in a free iron atom is:
A. 2
B. 3
C. 4
D. 6
Solution:
Electronic configuration of $\mathrm{Fe}:[\mathrm{Ar}] 3 \mathrm{~d}^6 4 \mathrm{~s}^2$
Accordina to Hund's rule. $3 d^6$ has 4 unbaired electrons.
Hence, the correct answer is option (C)
Frequently Asked Questions (FAQs)
The electronic configuration of an object is a symbolic representation of how the electrons of its atoms are distributed in different atomic orbital atoms. When writing an electron adjustment, a fixed notation is followed where the energy level and type of orbital are first recorded, followed by the number of electrons present in the orbital specified in the superscript. For example, the carbon suspension of carbon (atomic no of iron: 6) is 1s22s22p2.
The three laws that govern how electrons are filled with atomic orbitals are:
Aufbau principle: electrons must completely fill the atomic orbitals of a given energy level before settling in an orbital associated with a higher energy level. Electrons reside in orbital with a growing sequence of orbital energy levels.
The principle of Paul's release: states that no two electrons can have the same value in all four quantum numbers. As a result, each orbital subshell can accommodate 2 higher electrons and both of these electrons MUST have opposing spaces.
Hund's law of high duplication: All subshells in the orbital must be housed separately before any subshell can be doubled. In addition, the rotation of all electrons in individual subshells should be the same (to increase the total spin).
Electron alignment provides insight into the chemical behavior of objects by helping to determine the valence electrons of an atom. It also helps to separate items into separate blocks (such as s-block elements, p-block elements, d-block elements, and f-block elements). This makes it easier to study the properties of objects.
Electronic brass configuration by [Ar] 3d104s1. This adjustment does not comply with the aufbau goal due to the small power gap between 3d and 4s orbitals. The complete d-orbital filling provides more stability than the less-filled suspension.
When iron loses 2 4s electrons, it gains +2 valency. In some cases, the iron will lose even one of the paired electrons from the 3d orbital, leaving the entire 3d orbital full of unbroken electrons (providing stable stability). In this case, its valency will be +3.
