Acids and Bases - Definition, Theory, Features, Uses, FAQs
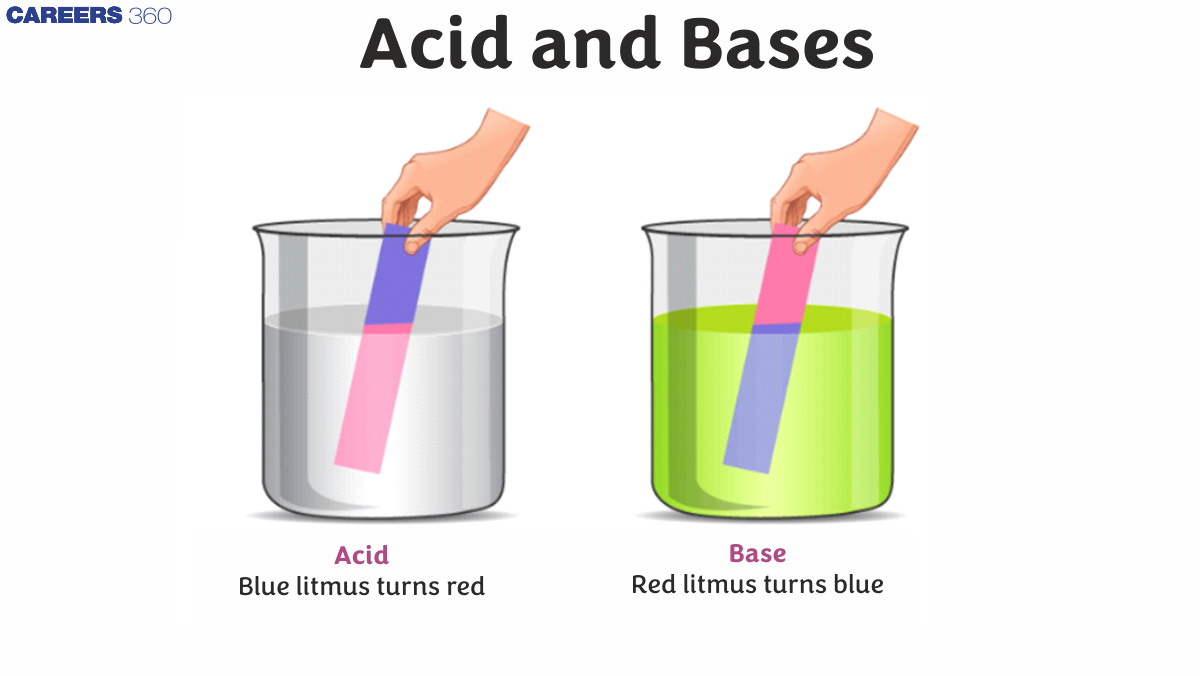
An acid is any substance that contains hydrogen that can donate a proton (hydrogen ion) to another substance. The base is a molecule or ion that is able to absorb hydrogen ions from an acid. Acid is basically a molecule that can donate $\mathrm{H}^{+}$ ions and can always release energy after the loss of $\mathrm{H}^{+}$ ions. Acids are known to turn red litmus to blue. The bases, on the other hand, are characterized by a bitter taste and a smooth texture. The base that can be dissolved in water is called alkali. When these substances react chemically with acids, they produce salts. The bases are known for turning red litmus into blue.
This Story also Contains
- Acid
- Acids and Bases Theories
- pH of Acids and Bases
- Acids and Bases
- The Arrhenius Concept of Acids and Bases
- Bronsted-Lowry's Theory of Acids and Bases
- Lewis Concept for Acids and Bases
- Use of Acids and Bases
- Some Solved Examples
Acid
Arrhenius first described acids as ionizing chemicals to produce hydrogen ions, and bases as ionizing chemicals to produce hydroxide ions. According to the Lowry-Bronsted definition, an acid is a proton donor and the basis for proton receptors.
According to Lewis's definition, acetic or ionic acids are able to bond with unrelated electrons, and bases are molecules or ions with a pair of unallocated electrons present to share with acids. To be acidic in Lewis's sense, the molecule must have an incoming electron. This is the general concept of an acid-base. All Lowery Bronstead acids are Lewis acids, but in addition, Lewis's definition includes many other reagents, such as boron trifluoride, aluminum chloride, etc.
Also read -
Acids and Bases Theories
Three different theories are set out to define acids and bases. These ideas, including Acids and bases, can be explained by three different perspectives.
- Arrhenius' theory of acids and bases usually states that "acid produces H+ ions in solution and bases produce OH- ions in their solution".
- The Bronsted-Lowry theory defines "acid as a proton donor and base as a proton acceptor".
- Finally, Lewis's definition of acids and bases defines "acids as electron-pair receptors and bases as electron-pair donors".
Examples of acids and bases are vinegar, citric acid, sulphuric acid and sodium hydroxide, calcium hydroxide, and milk of magnesia, respectively
pH of Acids and Bases
The pH scale (where pH stands for 'hydrogen energy') can be used to determine the numerical value of an acid level or the base of an object. A pH scale is a common and reliable way to measure what is acidic or basic. The pH ratio can vary from 0 to 14, where 0 is the most acidic, and 14 is the most basic. Blue litmus paper always turns red under acidic situations, and red litmus paper usually turns blue under basic or even alkaline conditions.
Acids and Bases
1. Features of Acids
- Acids are destructive to nature.
- They are good electric drivers.
- Their pH values are always below 7.
- When made of metal, these substances produce hydrogen gas.
- Acids are substances that have a sour taste.
Examples of Acids:
1)Hydrochloric acid [HCl],
2)Acetic acid [CH3COOH].
2. Features of Bases
- Some properties, such as bitter taste, are held by all the bases. The bases feel smooth, too. Dream about what a smooth soap looks like. And this is the foundation. In addition, when installed in water, the foundations conduct electricity because they contain particles charged to the solution.
- These substances usually release hydroxide ions i.e., OH negative ions, when they are dissolved in water.
- In their aqueous solutions, the bases act as good electrical conductors.
- The pH values corresponding to the bases remain higher than 7.
- Bases have a bitter taste that has the ability to turn red litmus paper blue.
Examples: Sodium hydroxide [NaOH], milk magnesia [Mg(OH)2], calcium hydroxide
[Ca(OH)2].
3. Neutral Items
A neutral substance is acid-free or basic in structure, has the same amount of hydrogen and hydroxyl ions, and does not change color
The Arrhenius Concept of Acids and Bases
- Swedish scientist Svante August Arrhenius described acids as substances that increase the concentration of H+ ions in water as they dissolve in it.
- These protons continue to form hydronium ions in combination with water molecules.
- Another advantage of this theory is that it effectively describes the reaction between acids and bases that produces salt and water.
- An important limitation of Arrhenius' definitions of acids and bases is that they fail to explain how non-hydroxide ions form basic solutions when dissolved in water.
Bronsted-Lowry's Theory of Acids and Bases
- Bronsted-Lowry theory usually defines the acid as a proton donor.
- The basis is defined as the proton receptor (or H+ ion receptor) in this sense.
- Bronsted acids are broken down to produce protons and thus increase the concentration of H+ ions in the solution.
- Bronsted foundations, on the other hand, absorb water-soluble protons (solvent) to produce hydroxide ions.
- An important limitation of this theory is that it fails to explain how hydrogen-deficient chemicals exhibit acid properties, such as BF3 and AlCl3.
|
Related Topics Link |
Lewis Concept for Acids and Bases
- Lewis's definition of acid states that it is an orbital type with no electron and therefore, has the ability to accept an electron pair.
- The Lewis base is a type that contains two electrons and can therefore act as an electron-pair donor.
This theory does not include the hydrogen atom in its definition of acids and bases.
Lewis acids are naturally electrophilic, and Lewis Bases have nucleophilic properties.
- Examples of Lewis acids: Cu2+, BF3, and Fe3+.
- Examples of Lewis bases: F-, and C2H4 (ethylene).
Lewis acid adopts an electron pair from a Lewis base, creating a bonding process in process. The resulting element is known as the Lewis adduct.
However, it provides little understanding of the power of these acids and foundations.
One of the disadvantages of this theory is that it fails to explain the acid-base reaction that does not involve the formation of a covalent bond.
NCERT Chemistry Notes :
Use of Acids and Bases
Various uses of acids and bases are listed in this article.
1. Use of Acids
- Vinegar, a diluted solution of acetic acid, has various home applications. It is also used as a food preservative.
- Citric acid is an important component of lemon juice and orange juice. It can also be used for food storage.
- Sulfuric acid is widely used in batteries. Batteries used to start car engines usually contain this acid.
- Phosphoric acid is the main ingredient in many cold drinks.
2. Uses of Bases
- The production of soap and paper involves the use of sodium hydroxide. NaOH is also used in the construction of rayon.
- Ca(OH)2, also known as slaked lime or calcium hydroxide, is used for powder bleaching.
- Dry mixtures used for painting or decoration are made with the help of calcium hydroxide.
- Magnesium hydroxide, also known as milk magnesia, is widely used as a laxative.
- Ammonium hydroxide is the most important reagent used in laboratories.
- Any excess acid in the soil can be reduced by using slaked lime.
Also, check-
Some Solved Examples
Question 1: Sodium carbonate is a basic salt because it is a salt of
(a) strong acid and strong base
(b) weak acid and weak base
(c) strong acid and weak base
(d) weak acid and strong base
Solution :
Sodium carbonate $\left(\mathrm{Na}_2 \mathrm{CO}_3\right)$ is the salt of weak acid carbonic acid $\left(\mathrm{H}_2 \mathrm{CO}_3\right)$ and a strong base sodium hydroxide ( NaOH )
$2 \mathrm{NaOH}+\mathrm{H}_2 \mathrm{CO}_3 \rightarrow \mathrm{Na}_2 \mathrm{CO}_3+2 \mathrm{H}_2 \mathrm{O}$
The salts of weak acids and strong bases give basic solutions (or alkaline solutions) having a pH of more than 7.
Hence, the correct answer is option (d)
$\mathrm{OH}^{-}, \mathrm{RO}^{-}, \mathrm{CH}_3 \mathrm{COO}^{-}, \mathrm{Cl}^{-}$
Solution:
Conjugate acids of given bases are $\mathrm{H}_2 \mathrm{O}, \mathrm{ROH}, \mathrm{CH}_3 \mathrm{COOH}, \mathrm{HCl}$.
Their acidic strength is in the order
$\mathrm{HCl}>\mathrm{CH}_3 \mathrm{COOH}>\mathrm{H}_2 \mathrm{O}>\mathrm{ROH}$
Hence, basic strength is in the order : $\mathrm{RO}^{-}>\mathrm{OH}^{-}>$
$\mathrm{CH}_3 \mathrm{COO}^{-}>\mathrm{Cl}^{-}$
Question 3: Which of the following salts will produce a basic solution in water?
A. NaCl
B. NH4Cl
C. NaCN
D. KHSO4
Solution:
$\mathrm{NaCl} \rightarrow$ neutral (strong acid + strong base)
$\mathrm{NH}_4 \mathrm{Cl} \rightarrow$ acidic (weak base + strong acid)
$\mathrm{NaCN} \rightarrow$ basic (strong base $\mathrm{NaOH}+$ weak acid HCN )
$\mathrm{KHSO}_4 \rightarrow$ acidic
Hence, the correct answer is option (c)
Frequently Asked Questions (FAQs)
Common acids include citric acid (found in citrus fruits), acetic acid (vinegar), and carbonic acid (in carbonated drinks). Common bases include baking soda (sodium bicarbonate), soap, and household cleaners containing ammonia.
A strong acid completely dissociates into its ions in solution, resulting in a high concentration of H⁺ ions (e.g., hydrochloric acid). A weak acid only partially dissociates in solution, resulting in a lower concentration of H⁺ ions (e.g., acetic acid).
An acid is a substance that donates protons (H⁺ ions) in a solution. Acids typically have a sour taste and can conduct electricity. Common examples include hydrochloric acid (HCl) and sulfuric acid (H₂SO₄).
To determine whether substance is acid or a base, count hydrogens on each substance before as well as after reaction. If the number of hydrogens has decreased, that substance is the acid. If the number of hydrogens has increased that substance is the base.
The solution contains more hydrogen ions than hydroxide ion. That solution is acidic. The foundation is something that can eliminate hydrogen ions. As the base dissolves in water balance between hydrogen ions as well as hydroxide ions changes on the other side.
Some examples are caustic soda or sodium hydroxide, calcium hydroxide or limewater, borax. The bases are classified as proton receptors (H+).
Acids are an ionic compound that, when dissolved in water, produces fine hydrogen ions (H+) when dissolved in water, the acid tastes acidic, conducts electricity and reacts with metals to form hydrogen gas. Certain reference chemicals can be used to obtain acids, such as litmus. Acids turn blue paper into a red litmus.
Two types of destructive elements are acid and bases. Any substance with a pH value between 0 and 7 is known to be acidic while the pH value between 7 and 14 is basic. Acids are ionic compounds that break up to form hydrogen ions (H+) in water.
