Clemmensen Reduction - Examples, Explanation, Applications, FAQs
Here, In this article will discuss clemmensen reduction, clemmensen reduction reaction, clemmensen reagents (Zn-Hg in Conc. HCl) used in the reaction, clemmensen reduction mechanism. Clemmensen reduction reaction was first reported by Clemmensen of Park Davis in 1913 and it was named after Eril Christian Clemmensen, a Danish chemist. At first, in 1913 E.
In Clemmensen reduction, Clemmensen described those simple ketones and aldehyde were converted to the corresponding alkanes upon refluxing for several hours with 40% aqueous HCl, amalgamated zinc and a hydrophobic organic co-solvent such as toluene. The reaction type of clemmensen reduction reaction is organic redox reaction.Reduction of ketones gives alkanes or saturated compounds, Below is Clemmensen reduction examples
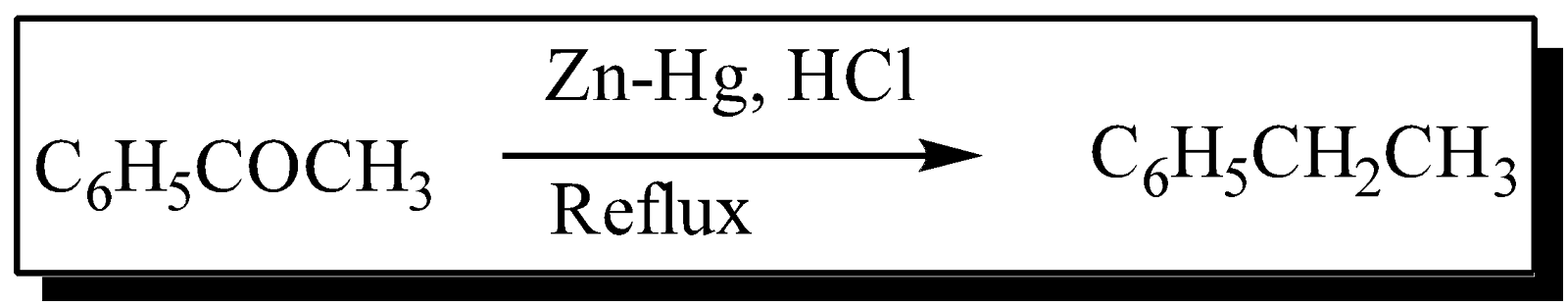
In Clemmensen reaction, the reduction of carbonyl groups of aldehydes and ketones to methylene groups with Zn-Hg in HCl is known as clemmensen reduction and this is clemmensen reagent. The initial procedure is rather harsh thus the Clemmensen reduction of acid-sensitive substrates and polyfunctional ketones is rarely successful in producing the expected yield.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
The clemmensen reduction reaction in Class 12 explains and involves in refluxing the carbonyl compounds treated with amalgamated zinc and excess of concentrated hydrochloric acid. The clemmensen reaction is useful mainly for ketones having phenolic or carboxylic groups which remain unaffected and Zinc amalgam formula is Zn-Hg. The reaction involves more reduction of ketones often than aldehydes as ketone is more reactive than aldehydes.
This type of reduction reaction is also seen in Wolff-Kishner reduction but Clemmensen reduction easier than this form product and to perform. The Clemmensen reduction fails with acid-sensitive and high molecular weight substrates and the Clemmensen reaction also shows reduction of α, β- unsaturated ketones which undergo reduction of both the olefinic and carbonyl groups. Buthowever, the reduction is specific for carbonyl groups of aldehydes and ketones containing other functional and reducible groups.
The Clemmensen reduction reaction of ketones is mainly productive at reducing aryl-alkyl ketones such as formed in the Friedel- Crafts acylation reaction. Mostly, it is used to convert acyl benzenes from Friedel-Crafts acylation to alkylbenzenes. Clemmensen reduction reaction involves Zn Hg HCl as clemmensen reducing agent and Yamamura and co-workers have reported a milder procedure which uses organic solvents (THF, Et2O, Ac2O, benzene) saturated with dry hydrogen halides (HCl, HBr) and activated zinc dust at ice-bath temperature and compared to the original Clemmensen reduction can be explained by procedure these modified reductions are complete with an hour at 0̊C and appropriate for acid and heat sensitive compounds.
In some case, some compounds have very low solubility in the usual solvents used for the clemmensen reaction, thus in these cases a second solvent like acetic acid, ethanol, or dioxane are added to the reaction mixture to increase the solubility of the substrate and allow the reduction reaction to take place and this is clemmensen reduction application. Most of the time mixtures are formed in these reactions, which contain a substantial amount of rearranged products.
Clemmensen Reduction Mechanism Explanation:
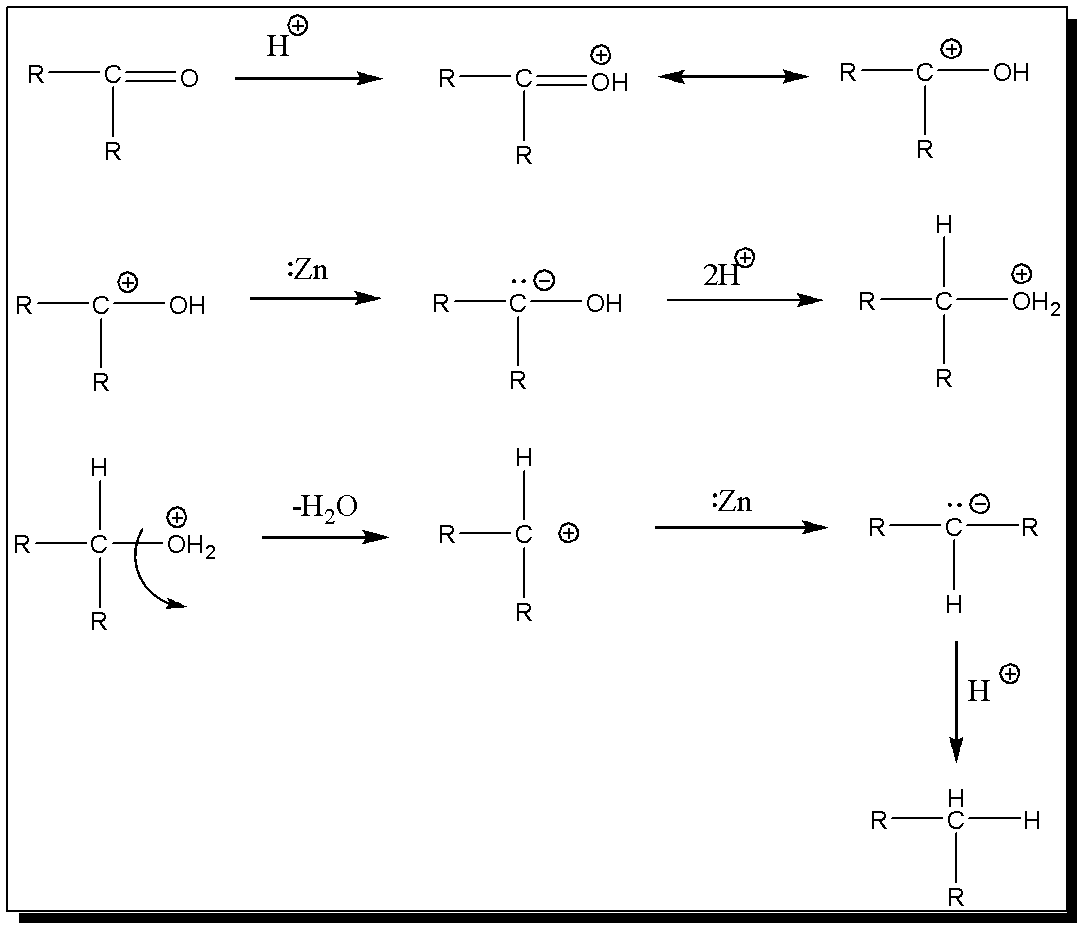
To explain clemmensen reduction, many mechanisms have been suggested which are so contradictory that no conclusion can be drawn. The mechanism where the intermediate formation of alcohol was rejected since the reagents fail to reduce most alcohols to hydrocarbons. Then, Nakabayashi has proposed a mechanism on the assumption that the reduction reaction under acid condition involves protonated carbonyl group to which electrons are transferred from the metal.
Here, Clemmensen reduction carbonyl compound is treated with zinc amalgam in HCl which is clemmensen reduction reagent to give alkanes. In one of the mechanisms the rate determining step involves the attack of zinc and chloride ion on the carbonyl group and the very important intermediates are carbanions, whereas in the other heterogeneous process, the formation of a radical intermediate and then a zinc carbenoid species is reported.
| Related topics link, |
The mechanism can be explained by the fact that the products formed in the various reductions are different when the reaction conditions ( e.g. concentration of the acid, concentration of zinc in the amalgam i.e. Zn-Hg HCl) are changed. It is also noted that the reduction occurs with zinc but not with other metals of comparable reduction potential.Clemmensen reduction carbonyl compound is treated with zinc amalgam in HCl which is clemmensen reduction reagent to give alkanes.
Certain type of clemmensen reduction of ketones and aldehydes and some other do not give the normal reduction products alone and hence α-hydroxy ketones give either ketones through hydrogenolysis of OH groups or olefins and 1,3-diketones give exclusively mono ketones with rearrangement in clemmensen reduction reaction.Many cyclic 1,3 – diketones give Clemmensen reduction a fully reduced product along with a mono ketone compound with ring contraction as when 5,5-dimethyl cyclohexane -1,3-dione treated with Zn-Hg in HCl gives 1,1 –dimethyl cyclohexane and 2,4,4-trimethyl cyclopentanone.
Also Read:
Synthetic Applications of Clemmensen reduction:
Many heterocyclic 1,3-dicarbonyl compounds possessing alkyl substituents at the electronegative 2-position exhibit interesting biological properties.
The synthesis of these compounds is either difficult or seeks for expensive starting/ initial materials.
T. Kappe and co-workers have found a simple and effective method for the reduction of acyl substituted 1,3-dicarbonyl compounds to the corresponding alkyl derivatives.
For example, 3-acyl-4-hydroxy2(1H)-quinolones and 3-acyl-4-hydroxy-6-methypyran-2-ones were reduced in good yields to 3-alkyl-4-hydroxy2(1H)-quinolinones and 3-alkyl-4-hydroxy-6-methylpyran-2-ones, respectively, using zinc powder in acetic acid/hydrochloric acid that is follows Clemmensen reduction reaction.
S.M. Weinreb and co-workers were amazed to find that the convergent stereoselective synthesis of marine alkaloid lepadiformine resulted in a product that gave a totally different NMR spectra than the natural product.
This finding led to the revision of the proposed structure of lepadiformine drug and in the last stages of the synthesis, they exposed a tricyclic piperidone intermediate to Clemmensen conditions to remove the ketone functionality.
Under these conditions the otherwise minor elimination product (alkene) was formed majorly; however, it was possible to hydrogenate the double bond to give the desired alkane by using Clemmensen reduction.
In the laboratory of F.J.C. Martins the synthesis of novel tetracyclic undecane derivatives was made. In one of the middle synthetic sequences the Clemmensen reduction was used to remove a ketone functionality in good yield to form the subsequent desired product.
Clemmensen reduction is used for the reduction of aliphatic and mixed aliphatic – aromatic carbonyl compounds. The clemmensen reaction is very useful for introducing straight –chain ( without rearrangement) alkyl groups in aromatic rings by acylation and subsequent reduction reaction.
Clemmenson reduction reaction is used for reduction of keto acids as example but however, α, β- keto acids are generally not reduced. β-Benzoyl propionic acid in presence of Zn-Hg in Conc HCl gives γ-Phenyl butanoic acid.
It is used for the reduction of phenolic carbonyl compounds. Salicylaldehyde in presence of Zn-Hg in HCl gives o-Cresol.
Clemmensen reaction is used in the synthesis of naphthalene.
Reduction of ring expansion such as 1-Methyl-2-propionyl pyrrolidine in presence of Zn-Hg in HCl to form 2-Ethyl-1-methylpiperidine.
During the enantioselective total synthesis of denrobatid alkaloid (–)-pumiliotoxin C by C. Kibayashi et al., an aqueous acyl nitroso Diels-Alder cycloaddition was used as the key and very important step. In the end of the total synthesis, the cis-fused decahydroquinolone was made to the Clemmensen reduction conditions to give a 2:1 epimeric mixture of deoxygenated products in 57% yield which is moderate in yield. Any Subsequent debenzylation converted the major isomer into 5-epi-pumiliotoxin C alkaloid.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
