Enthalpy Change
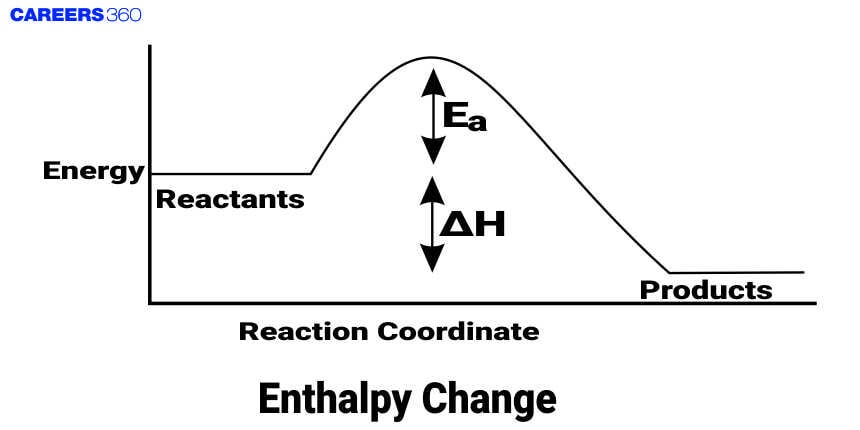
The change in enthalpy is defined as ΔH and is a central concept in thermodynamics. Enthalpy change is described as the change in heat content of a system for a process occurring at constant pressure. Anyway, enthalpy is a state function. Enthalpy change can be plus or minus. A positive ΔH means that an endothermic reaction is involved, in which heat goes from the surroundings to the system. On the contrary, a negative ΔH is known as an exothermic reaction, meaning that heat is being given out into the surroundings from the system. Enthalpy change forms a very vital part of studies in reaction energetics and rules reaction spontaneity; it plays a major role in combustion, phase transitions, and biochemical reactions. It is measured in units of energy, usually joules, J, or kilojoules, kJ.
This Story also Contains
- Heat of Formation
- Some Solved Examples
- Summary
Heat of Formation
The amount of heat evolved or absorbed or changed in enthalpy when 1 mole of a substance is obtained from its constituents or free elements.
Once the value of Ho at 25o C for any species has been assigned the value of Ho at other temperatures Can be found out by using Kirchoff's equation as follows.
1. Standard heat of formation of a free element is taken as zero.
For example, In carbon—graphite form is taken as the standard state, and in sulphur, the monoclinic form is the standard state.
2. The heat of formation may be +ve or -ve.
If
If
3. The stability of the exothermic compound is more than that of the endothermic compound hence, the greater the liberated energy greater the stability of the compound.
Example, HF > HCI > HBr > HI
Recommended topic on video(Enthalpy Change)
Some Solved Examples
Example 1: What is the standard enthalpy of formation of O2 gas?
1)Positive
2)Negative
3) Zero
4)Can be positive or negative both
Solution
The standard enthalpy of formation for an element in its standard state is ZERO because elements in their standard form are not formed, they are naturally in that state. As the natural state of Oxygen is gas hence enthalpy of the formation of Oxygen gas is zero.
Hence, the answer is the option (3).
Example 2: Which of the following forms of carbon has zero standard enthalpy of formation?
1) Graphite
2)Diamond
3)Fullerene
4)None of the above
Solution
The standard enthalpy of formation for an element in its standard state is ZERO because elements in their standard form are not formed, they are naturally in that state.
Hence, the answer is the option (1).
Example 3: Based on the following thermochemical data :
The value of enthalpy of formation (in kJ) of OH- ion at 25°C is:
1)-22.88
2) -228.88
3)228.88
4)-343.52
Solution
Hence, the answer is the option (2).
Example 4: The enthalpy changes for the following processes are listed below:
Given that the standard states for iodine and chlorine are I2 and Cl2(g) , the standard enthalpy of formation (in kJ mol-1) for ICl(g) is:
1)-14.6
2)-16.8
3) 16.8
4)244.8
Solution
As we learned from the concept
Hence, the answer is the option (3).
Example 5: The standard heat of formation
1) -192.5
2)192.5
3)- 292.5
4)292.5
Solution
The reactions we have:
Multiply eq(ii) by 2 and eq(iii) by 3.
Now,
Thus, the equation is given as:
Hence, the answer is the option(1).
Summary
Enthalpy change, ΔH, is the heat transferred during a chemical reaction at constant pressure. It measures the change in enthalpy of products minus reactants. Positive ΔH means it's an endothermic reaction where heat is absorbed, and negative ΔH means it's an exothermic reaction where heat is given off. It is, therefore, an important concept in understanding the dynamics attributed to the energy of reactions, methods for the prediction of reaction behavior, and the design of industrial processes. The change in enthalpy is expressed in joules, J, or kilojoules, kJ. It is a very basic value in chemistry, environmental science, and engineering. The study of changes in enthalpy allows chemists to make predictions about whether a reaction will be energy-releasing or energy-absorbing, cardinal in practical applications.
