Formic Acid - Structure, Properties, Natural Occurrence, FAQs
Formic acid is the simplest carboxylic acid. HCOOH is the molecular formula for methanoic acid. Formic acid’s HCOOH IUPAC name or HCOOH name is methanoic acid. The acid has a single carbon atom, two oxygen atoms, and two hydrogen atoms, as shown in the formula. It's a key intermediate in chemical synthesis that can be found in nature, most notably in ants. The term "formic" is based on the Latin formica, which refers to the substance's early extraction via ant body distillation.
This Story also Contains
- Methanoic Acid structure
- Physical Properties
- Chemical Properties
- Natural Occurrence
- Manufacturing Processes for Formic Acid
- Formic Acid Uses
Esters, salts, and the anion formed by formic acid are known as formates. In industry, methanol is used to make formic acid. Although formic acid is not extensively utilized as a solvent, it is interesting as a protic solvent with a high acidity. Corrosive and skin sensitizers, formic acid and its salts. The eyes are somewhat irritated by sodium formate. Formylic acid, and Aminic acid are some of its other names of formic acid.
This article covers formic acid sources ,other names of formic acid ,formic acid uses , formic acid structure , formic acid preparations, chemical formula of formic acid etc.It also discusses properties like density of formic acid , molecular weight of formic acid etc.
Also read -
Methanoic Acid structure
Formic Acid has a simple structure because it is the first carboxylic acid in the series and includes only one carbon atom, giving it the moniker methanoic acid. A carbon atom has a single bond with hydrogen, a double bond with oxygen, and another single bond with oxygen that is connected with a hydrogen atom in the structure.
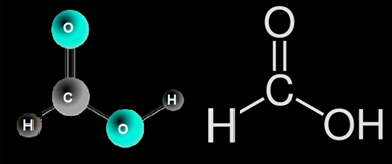
Lewis structure of formic acid
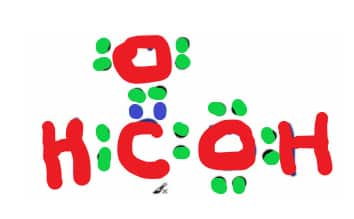
Properties
Physical Properties
The IUPAC designation for Formic acid is Methanoic acid. It is the first of the carboxylic acids in the homologous series.
Formic acid seems to be a thick translucent liquid with no discernible colour, making it difficult to identify.
Formic acid has a rather low melting point. The melting point of formic acid is only 8.4°C.
Formic acid has a somewhat higher boiling point than water. Formic acid has a boiling point of 100.8°C.
Formic acid has a low density of 1.22g/cm3 and is not a particularly dense liquid.
Because formic acid is the first in the homologous series, its molecular weight isn't particularly high. Methanoic acid has a molecular weight of 46.03 g/mol
The odour of formic acid is distinctively harsh and unpleasant.
It is water soluble and miscible.
Formic acid is colorless, flammable liquid with a sharp odor.
It is soluble in hydrocarbons and miscible with water and most polar organic solvents
Chemical Properties
Formic acid, as its name implies, is acidic and can convert blue litmus to red litmus
Formic acid is a donor of hydrogen bonds. It is made up of hydrogen-bonded dimers rather than individual molecules in hydrocarbons and the vapour phase. The ideal gas law does not apply to gaseous formic acid because of its ability to hydrogen-bond. Solid formic acid is made up of a practically infinite network of hydrogen-bonded formic acid molecules that can exist in one of two polymorphs

In most cases, formic acid comprises carbon and carbon types of covalent connections. Carbon forms all covalent bonds in formic acid as well. Only covalent bonds exist in formic acid
Mercuric chloride can be reduced to mercurous chloride by formic acid, resulting in a white precipitate. The reaction's equation is shown below.
HCOOH + 2 HgCl2 → Hg2Cl2 + 2HCl + CO2
With water, formic acid produces a high-boiling azeotrope (22.4 percent ). Formic acid in liquid form has a tendency to supercool.
Formyl chloride, phosphoryl chloride, and hydrogen chloride are generated when formic acid reacts with phosphoric pentachloride. The equation is shown below.
HCOOH + PCl5 → HCOCl + POCl3 + HCl
Natural Occurrence
Formic acid can be found in most ants and stingless bees of the species Oxytrigona in nature. Formic acid can be sprayed by Formica ants on their prey or to defend their colony. When challenged by predators, the puss moth caterpillar (Cerura vinula) will spray it as well. It's also present in stinging nettle trichomes (Urtica dioica). Because of forest emissions, formic acid is a naturally occurring component of the atmosphere.
Manufacturing Processes for Formic Acid
Formic acid is produced by the following methods. Formamide is formed by reacting methyl formate with formamide.Methyl formate is generated when methanol and carbon monoxide combine in the presence of a strong base. Below is the chemical reaction to the above-mentioned procedure.
CH3OH + CO → HCO2CH3
In industries, the procedure is also employed, and the reaction is carried out under particular conditions. The typical circumstances for the reaction's progress to be possible, are 80 °C temperature, 40 atm pressure,liquid phase.
Sodium methoxide is the most common base utilised in this method.The major result of hydrolysis of the obtained methyl formate is our target chemical. We also obtain various byproducts as a result of this. The following is the hydrolysis reaction
HCO2CH3 + NH3 → HC(O)NH2 + CH3OH
2 HC(O)NH2 + 2 H2O + H2SO4 → 2 HCO2H + (NH4)2SO4
However, the approach described above has a drawback. The ammonium sulphate that is produced as a by-product of the reaction must be disposed of. Because the chemical is dangerous and can cause significant environmental harm, disposing of it is a time-consuming operation. Since numerous governments have restricted the disposal of chemicals into the environment in order to safeguard it, this has caused many manufacturers challenges.
Also Read:
Formic Acid Uses
In a fuel cell, formic acid can be employed (it can be used directly in formic acid fuel cells and indirectly in hydrogen fuel cells).Because it is unable to eliminate iron oxide deposits on its own, it is mixed with citric acid or HCl.
Saturated monocarboxylic acids are used in a variety of industrial chemicals
To reduce sodium and potassium dichromate, formic acid is used as a reducing agent.
Useful in the dyeing and tanning industries, but other competitive acids have generally been cheaper, limiting the usage of formic acid to a few applications where it has distinct advantages.
Formic acid is widely used in cattle feed as a preservative and antibacterial agent
It is sometimes added to feed in the poultry business to destroy E. coli germs
Because of its acidic nature, formic acid is also widely employed in the manufacturing of leather, including tanning (23 percent of global use in 2009) and dyeing and finishing textiles (9 percent of global consumption in 2009)
Microbes can be used to manufacture isobutanol from carbondioxide by employing formic acid as an intermediate
In reversed-phase high-performance liquid chromatography (RP-HPLC) analysis and separation techniques for the separation of hydrophobic macromolecules such as peptides, proteins, and more complicated structures such as whole viruses, formic acid is frequently utilised as a component of the mobile phase. Formic acid has various advantages over the more commonly used phosphoric acid, especially when combined with mass spectrometry detection.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
