Hell Volhard Zelinsky Reaction Mechanism - Significance, Application with FAQs
In this article we will discuss about hell volhard zelinsky reaction (or hvz reaction in short), hell volhard zelinsky reaction mechanism( or hvz reaction mechanism), hell volhard zelinsky reaction example hvz reaction example, what hvz reaction is used to prepare, hvz reaction definition and everything related to hell volhard zelinsky reaction (or hvz reaction class 12).
Hell Volhard Zelinsky reaction
Nikolay Zelinsky in 1923
The name hell volhard zelinsky (or hvz) is named after three chemists. They were Carl Magnus von Hell; a German chemist, Nikolay Zelinsky; a Russian chemist, and Jacob Volhard; a German chemist. Hvz reaction is a chemical reaction used to halogenate α-carbon of carboxylic acids. Along with water, Phosphorus as a catalyst is used in the hvz reaction.
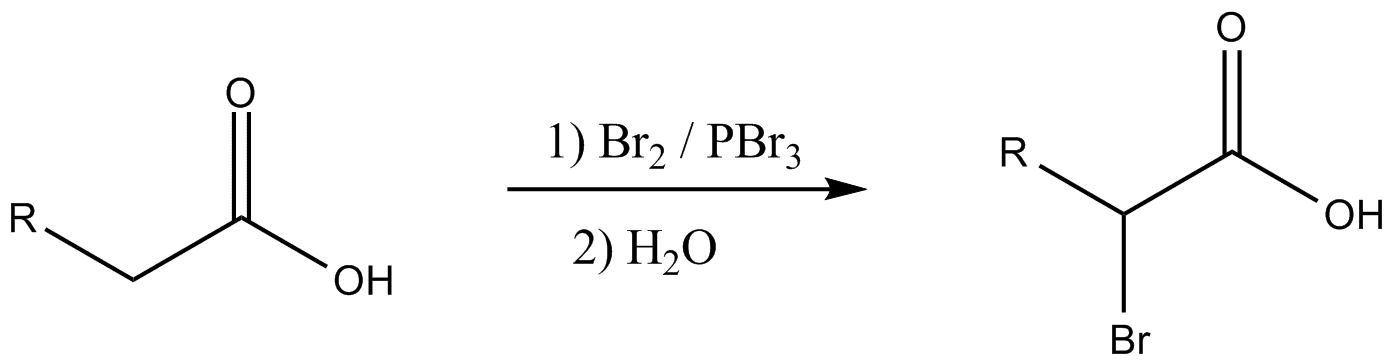
The catalyst used in hvz reaction
A catalytic amount of phosphorus tribromide PBr3 followed by one molar equivalent of bromide Br2. Tribromide is formed by the reaction of red phosphorus and bromine gas Br2. PBr3 is very reactive and readily reacts with water vapour present in the atmosphere to form P-O bond, when left out in air. In hvz reaction, PBr3 has the responsibility of converting carboxylic acid into acyl bromides.Br2 is added in excess amount in hvz reaction to form PBr3.
This is because PBr3 by itself is not sufficient enough to perform two duties; role as a catalyst and participate in halogenation. Addition of Br2 in excess amount, in hvz, ensures that there is at least one extra equivalent of Br2 available to carry out the addition of halogen at the α-carbon. Hvz reaction cannot be used for fluorination and bromination of carboxylic groups.
Also read -
Significance of hvz reaction
As we know, aldehyde and ketones with α-H can undergo electrophilic addition reaction to form an enol tautomer which is in equilibrium with the substrate aldehyde or ketone. However, this is not quite the case with carboxylic acids. Carboxylic acids generally do not form stable enols which mean addition at α C becomes difficult.
Thus, α addition is not as effortless for carboxylic acid as it is for aldehydes and ketones. This is where the requirement of hvz reaction comes into play. The significance of the hvz reaction is to form a derivative, by conversion of the carboxylic acid, which can undergo tautomerization and is capable of executing α addition reaction.
| Related topics link, |
Hvz reaction mechanism
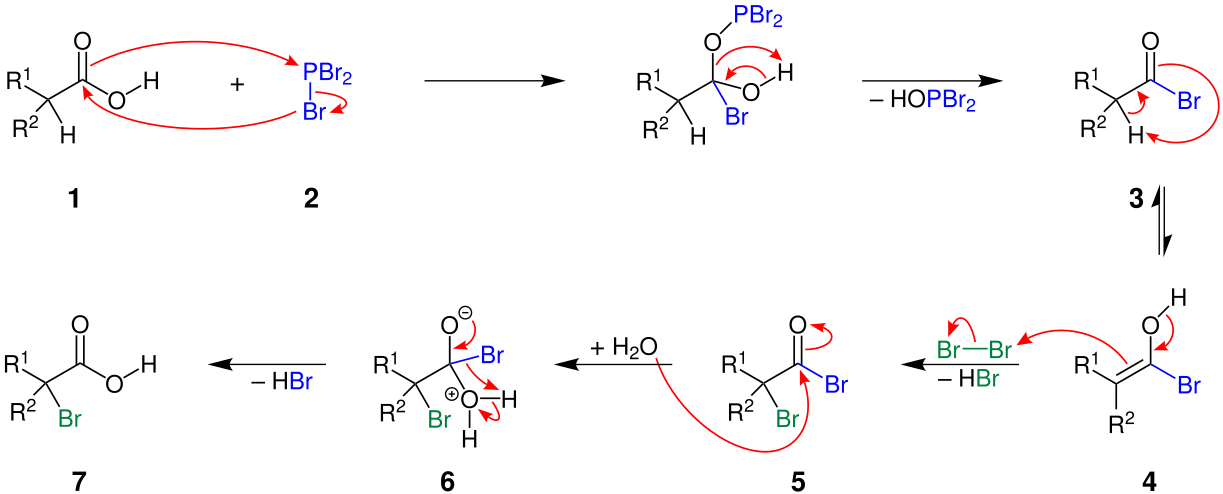
The hvz reaction initiates with addition of phosphorus tribromide. When PBr3 is added, oxygen of the carboxylic group attacks the phosphorus of PBr3 which results in breaking of C-O pi bond (1). This breaking of the C=O bond influences the hydroxyl OH group to become a good leaving group. With withdrawal of hydroxide group, a tetrahedral intermediate is formed (2). The orbitals of oxygen which were first employed to form pi bonds are now participating in bond formation with phosphorus.
The hvz reaction proceeds with deport of HOPBr2 from the previously formed intermediate to give an acyl bromide (3). The previous C-O bond in carboxylic acid is broken to form a new C-Br bond.
As we discussed previously, addition of halogen takes place at α-carbon. The carbon next to the carbonyl group is known as alpha carbon. Carbonyl compounds with α-carbon possessing α-hydrogen are capable of undergoing enolization and hence form enols. Enols are basically olefins with a hydroxyl group at one end (4).
The reaction moves forward with breaking of the carbon-oxygen pi bond present in acyl bromide. The formation of a new bond between carbonyl carbon and alpha carbon takes place.
This step of breaking of C-O pi bond and formation of C-C pi bond between acyl bromide (3) and enol form (4) is in equilibrium.
Since enols are nucleophilic in nature, they readily react with Br2 when Br2 is added in excess. In this step, halogenation takes place. The C=C bond breaks with addition of Br to the alpha carbon (5). This induces a positive charge on hydroxide oxygen which in turn results in expel of hydrogen. Overall, a molecule of HBr is removed in this step of the hvz reaction.
Hvz reaction comes to an end with hydrolysis of newly generated acid bromide (5) to form a carboxylic acid derivative. Water being a nucleophile, when added, attacks the carbonyl oxygen and attaches itself to the carbonyl carbon (6). This nucleophilic acyl substitution results in elimination of HBr and regeneration of neutral carboxylic group (7).
Also Read:
Application of hvz reaction
Hvz reaction is often used in preparation of alanine. Synthesis of various amino acids is also an important application of hvz reaction. The hvz reaction for synthesis of amino acids proceeds with displacement of halogens by using ammonia. An example of such hvz reaction is given below:
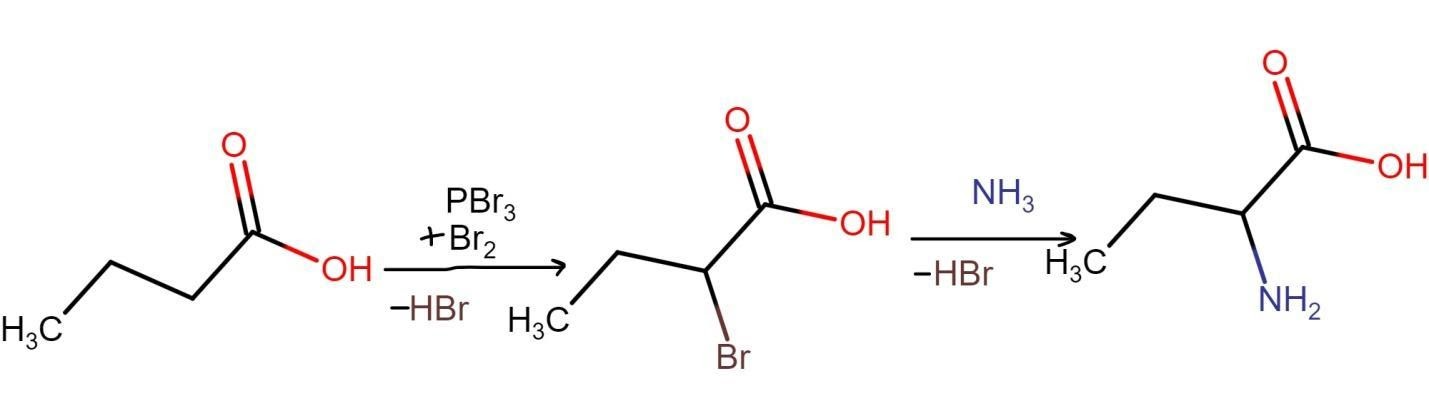
Alpha bromination of carboxylic acid remains the most important application of hvz reaction. (This article covers details of alpha bromination of carboxylic acid.)
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: