Isomers of Butane - Definition, Types, Structure with FAQs
How can two compounds contain the same number and type of atoms but have different properties and structures? The answer is isomerism. It is the phenomenon where compounds share the same molecular formula but differ in structure or arrangement, leading to unique physical and chemical properties. In this article, we will study the isomerism in butane. Butane generally shows structural isomerism. to know more, scroll down.
This Story also Contains
- Types Of Isomerism in Butane
- Structural Isomers Of Butane
- Isomers Of Butene
- Isomers Of Butyne
Types Of Isomerism in Butane
Constitutional isomers (Based on connectivity)
These are the isomers having the same chemical formula, but the atoms or groups of atoms differ in their connectivity.
There is a possibility that butane can have two different structures based on connectivity. There can be straight-chain butane as well as branched-chain butane. Both these structures of butane fulfill the valency of the carbon atom and thus form four bonds. There are 13 total numbers of covalent bonds in butane as well as isobutane.
The name isobutane came from the fact that it is a constitutional isomer of butane. The structural unit of the ‘iso’ group is the carbon atom bonded to hydrogen and two -CH3 groups. This is also known as chain isomerism. These are the isomers that differ in the skeletons of carbon atoms.
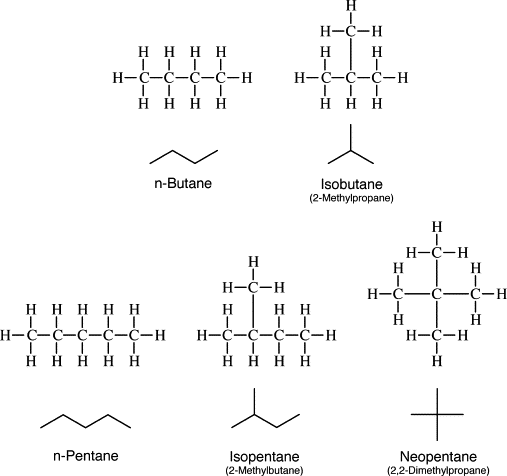
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Conformational isomers (Based on rotation around a sigma bond)
Different spatial arrangements of atoms in molecules can be obtained due to free rotation around sigma bonds. All these arrangements are known as conformations. The carbon-carbon bond in butane is a sigma bond; therefore, rotation around the carbon-carbon single bond is possible. This rotation around the C-C single bond can occur without causing any effect on the overlap of orbitals.
A large number of spatial arrangements is possible due to this rotation. However, this rotation is not totally free. An energy barrier of about 1-20 kJ mol-1 has to be crossed to overcome the torsional strain. This torsional strain arises due to the repulsive forces between electron pairs. Two types of conformations are possible-
Eclipsed conformation- In this conformation, the groups around the sigma bond are directly behind those of the other. A conformation can either be fully eclipsed or partially eclipsed. According to the same difference, the energy of a conformation can increase or decrease.
Staggered Conformation- In this conformation, the groups around the sigma bond are staggered with respect to each other. Staggered conformation is of two types, anti and gauche conformation.
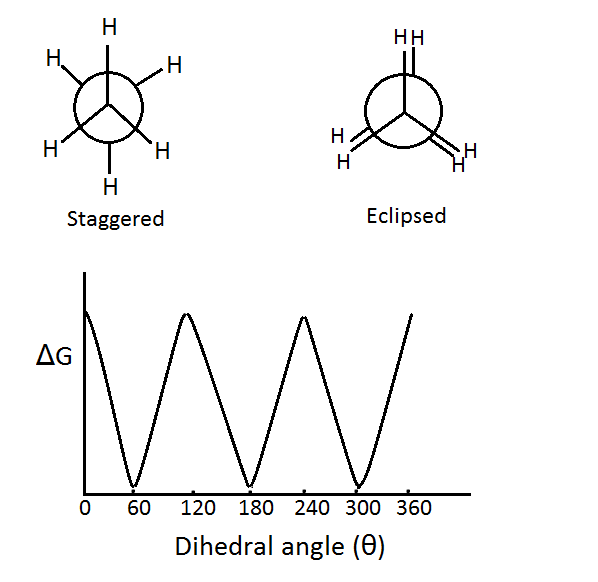
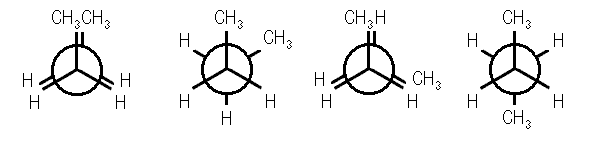
Four conformations are possible for butane, namely anti conformation, Gauche or skew conformation, eclipsed conformation, and fully eclipsed conformation. When the rotation takes place about the C2-C3 bond, the conformation with the two methyl groups opposite to each other (or 180⁰ away from each other) is called anti conformation and is maximum staggered.
Anti Conformation Of Butane
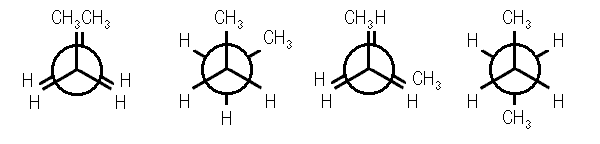
With a rotation around the C2-C3 at an angle of 60⁰, this conformation becomes eclipsed; further rotation about an angle of 60⁰ will give skew conformation.
Eclipsed Conformation Of Butane
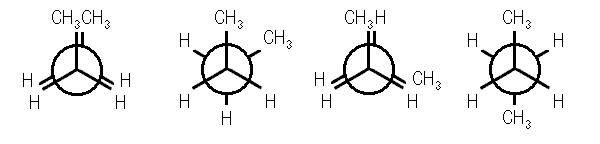
Gauche Conformation Of Butane
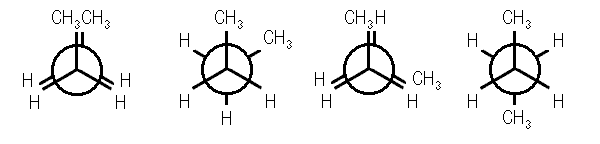
This conformation can also be staggered; however, the methyl groups will be comparatively closer. Hence, there will be repulsions between the electron pairs which will lead to an increase in the energy of gauche conformation by 3.8 kJ/mol. A fully eclipsed conformation is the most unstable conformation because the methyl groups are closest to each other. This fully eclipsed conformation has the highest energy and 19 kJ/mol more energy than the anti-conformation.
Fully Eclipsed Conformation Of Butane
Structural Isomers Of Butane
There are two possible isomers of butane.
.png)
N butane formula: C4H10
.png)
Isobutane formula: C4H10
IUPAC name of isobutane is 2-methylpropane. That means the 2-methylpropane structure formulas are the same as the isobutane structural formulas.
Both isobutane and n-butane are C4H10 isomers.
To understand isomerism better, let’s examine more compounds that show isomerism.
Isomers Of Butene
There are four possible isomers of butene.
Butene molecular formula: C4H8
The structures of the four possible isomers of butene are:
.png)
but-1-ene structure
.png)
Trans-2-butene structure
.png)
cis-2-butene structure
.png)
Isobutene structure
Where cis-2-butene and trans-2-butene are geometric isomers of 2-butene. Here, but-1-ene and but-2-ene (both cis and trans) are position isomers of butene. But-1-ene and but-2-ene (both cis and trans) and isobutene are structural isomers of butene.
Understanding the isomerism in but-2-ene-
Butene structure has four carbon atoms in which two carbon atoms are connected through a double bond. Just like how conformational isomerism was possible due to free rotation around the carbon-carbon sigma bond. Another type of isomerism is possible as a result of restricted rotation around a carbon-carbon double bond called geometrical isomerism.
Isomers Of Butyne
There are 2 isomers of butyne. 1-butyne structural formula and 2-butyne structural formula is given as:
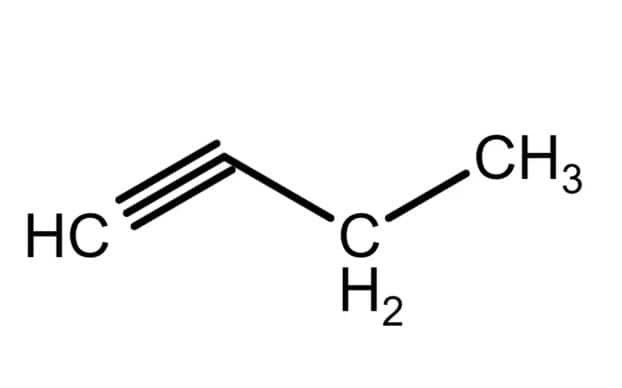
1-butyne
.png)
2-butyne
There are 35 isomers of nonane and 75 isomers of decane.
Practice Questions With Link Given Below
| Isomerism practice questions and MCQs |
| Nomenclature and Isomerism of Alkenes practice questions and MCQs |
Also Read:
Frequently Asked Questions (FAQs)
D) butane and isobutane
A) The isomer is 2-methylbutane
B) The isomer is n-pentane
C) The isomer is neopentane
N butane and isobutane are chain isomers and show structural isomerism.
Two isomers of butane can exist
Isomers of a substance must have the same molecular formula.
13
isobutane is an isomer of butane.
n-butane and isobutane
2-butyne and 1,3-butadiene are chain isomers.
Methylpropane is an isomer of n butane
