Xylene - Uses, Formula, Structure, FAQs
Xylene or dimethyl benzene is a highly flammable colorless liquid with a chemical formula (CH3)2C6H4. As the name dimethyl benzene suggested, it is a compound where the benzene ring has been substituted by 2 methyl groups. It is a colorless liquid with slightly greasy liquids with a very important use industry. Xylene was first discovered from a constituent of wood tar by a French chemist named Auguste Cahours. Xylene is produced industrially by the methylation reaction of toluene and benzene. Xylene is also produced by the process of catalytic reforming and by coal carbonization.
This Story also Contains
- Xylene formula
- Xylene Structure
- Side effects of Xylene
- Xylene uses
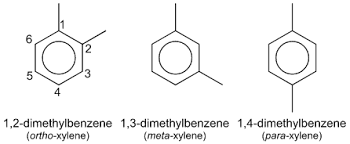
There are three isomers for xylene ortho-xylene, meta-xylene, and para-xylene. Ortho-xylene name is 1, 2 dimethyl benzene here the methyl groups are substituted on the ortho positions of the benzene ring. And the name of meta-xylene is 1, 3 dimethyl benzene where the methyl groups are substituted on the meta position of benzene. And para toluene with the name 1, 4 dimethylbenzene where the methyl groups are substituted on the para position of the benzene ring. Of the three isomers of xylene, they all have the same chemical formula C8H10.
The mixture of xylene is mainly used as a solvent and it is a bit greasy and has a sweet smell. Mixture xylene contains xylene and also ethylbenzene. It is used as a solvent within the manufacture of the many chemicals, sprays, coatings, and adhesives and as an ingredient in aircraft fuels and types of gasoline. Xylene is also used as a feedstock for the production of various polymers. Gasoline contains 1 and 6% of xylene. It is also present in paint together with toluene. Xylene is also found naturally in petroleum together with benzene, ethylbenzene, and toluene.
The molecular weight of xylene is 106.16 grams per mole with a density of 0.864 grams per ml. The boiling point and the melting point of xylene are 138.5 degrees Celsius and -47.4 degrees Celsius. Xylene boiling point is -47.4 degrees Celsius.
Also read -
Xylene formula
The chemical formula of xylene is C8H10.
Xylene Structure
Xylene structure can be explained in this portion. As xylene exists in three isomeric forms is ortho-xylene, meta-xylene, and para-xylene, it has three different structures also with the same chemical formula C8H10. For 1, 2 dimethyl benzene the second methyl group is attached nearby to the carbon atom where the first methyl group is attached and hence the name ortho-xylene since it is the ortho position of a benzene ring.
For 1, 3 dimethylbenzene the second methyl group is attached to the third position from the carbon atom where the first methyl group is attached and therefore the name xylene since it is the meta position of the benzene ring. For 1, 4 dimethylbenzene the second methyl group is attached to the fourth position of the first methyl group attached, and therefore its name is para-xylene as it is the para position of the benzene ring.
Para xylene has much industrial application as it can be easily oxidized to terephthalic acid also. The following figure shows the structure of ortho, meta, and para-xylene.
Side effects of Xylene
Xylene has a very adverse effect on human beings. Approximately 60 percent of airborne xylenes are easily absorbed to the lungs and thereby it then leads to the bloodstream and causes several damages to our body. It is also readily absorbed through broken or intact skin. Due to exposure to the environment xylene is found in fruits and vegetables and it then easily enters our body and causes serious problems. Other food items like meat products, eggs, milk, bread, potatoes, poultry, nuts, etc. also contain a trace amount of xylene.
This absorbed xylene tends to distribute it to many tissues in the body thereby causing problems to the tissues even when it enters the fetal tissues. But the xylene is easily excreted through the urine by converting it into a water-soluble metabolite that is, approximately 95% of absorbed xylene is excreted through the urine and thereby reducing the chance of risk. And it is not yet discovered whether it causes harm to the fetus.
It affects the brain to a great extent and high levels for short or long periods will cause headaches, lack of muscle coordination, dizziness, confusion, etc. It can also cause difficulty in breathing, memory difficulties, stomach discomfort, and affects the liver and kidneys. And at a high level leads to unconsciousness and even death.
Related Topics link, |
Xylene uses
- Para xylene is used for the production of terephthalic acid and dimethyl terephthalates and these two are used for the production of PET (polyethylene terephthalate).
- Ortho xylene is used for the preparation of phthalic anhydride.
- Xylene requires only slow drying so it has been substituted with toluene.
- Xylene is a very good clearing agent.
- Xylene has been used for the removal of paraffin from dried microscope slides before staining.
- It is commonly used as a solvent within the printing, leather, and rubber industries.
- It is used as a cleaning agent in steel and electronic components like silicon wafers.
- It is widely used as a solvent in paint since it is excellent in removing old paints from the surfaces.
- It is also used as a substitute over other paint thinning agents like toluene. This is due to the reason that xylene does not evaporate in comparison to toluene.
- It is also used in medical applications mainly in the dental field.
- It is used as a lubricant in many sectors.
- Xylene is one of the major constituents in petroleum lubricants, hair thinners, waxes, adhesives, petrol, tobacco, polishes, etc.
- For reducing the temperature of reaction vessels used in the laboratory xylene is used.
- Xylene was used as a tear gas agent in world war.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 13 Hydrocarbons
- NCERT notes Class 11 Chemistry Chapter 13 Hydrocarbons
Ozonolysis of O-xylene
The oxidative cleavage of an unsaturated bond in a compound by the reaction with ozone is ozonolysis. And therefore the end product will be a double bond replaced with a bond to an oxygen atom. In the case of o-xylene, there are two resonance structures present. The first resonance structure is shown below.
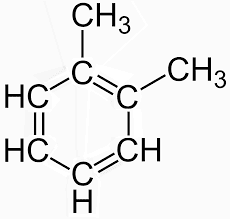
Resonance structure of o-xylene
And the second structure is shown below.
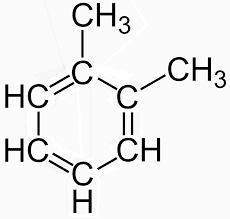
Resonance structure of o-xylene
The above shown figure is of xylene structure. As two structures are present two different types of compounds will also be produced. By the reaction with the first structure glyoxal and methyl, glyoxal is formed. While the reaction with the second structure forms glyoxal and dimethyl glyoxal.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
