Polyester in Chemistry: Synthetic Fibers, Textiles and Clothing
Polyester is a name that has come to be used very closely in our everyday lives; often, we do not even realize the same. From the garments we wear and the upholstery on chairs to a variety of other domestic and personal uses, polyester has woven itself into the very fabric of modern existence. Now think about slipping into a comfortable, non-wrinkled shirt that still holds its shape after many washes, or maybe leaning on that soft, durable couch that's strong enough to withstand family life. Much of this is owed to polyester, the synthetic fiber that changed the textile industry.
This Story also Contains
- Understanding Polyester
- Types and Characteristics of Polyester
- Relevance and Real-life Application
- Some Solved Examples
- Summary
However, polyester's impact goes far beyond fibers. It found its way into various aspects of our everyday lives, from the plastic bottles people utilize to the insulation materials keeping our homes nice and warm. Innovation, versatility, and adaptability have written a story in life for polyester. Being a type of polymer characterized as containing the ester functional group, with time, polyester has evolved into different types for fitting a particular need.
Understanding Polyester
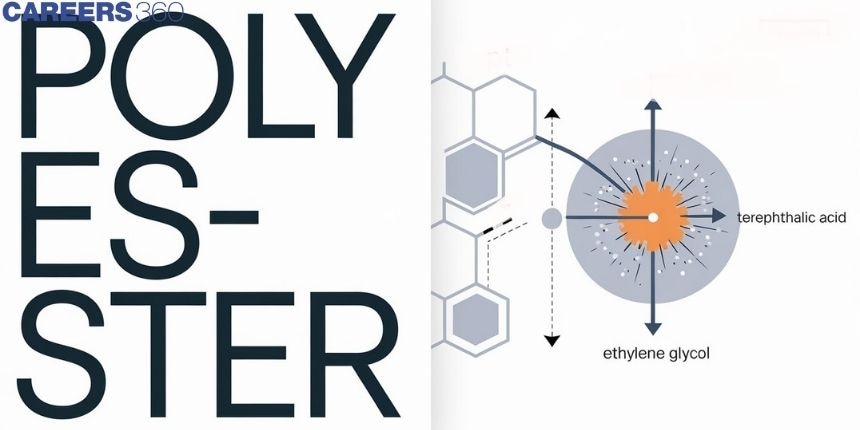
Polyester is an ester of polymerization. The latter term, more popularly, is an epitome of a synthetic fiber made from polyethylene terephthalate, obtained from petroleum products. It is during the process of polymerization that polyester emerges by linking ethylene glycol and terephthalic acid together into one material. It is possible to fabricate them by spinning them into fibers or by molding them into shapes. In this way, the fibers taken out are strong, resistant to shrinking or stretch, and quick to dry. So polyester serves in everything from clothing to industrial materials. On top of this, it can be blended with natural fibers like cotton and still come as really good at textile comforts. Further, as we discuss the topic of polyester, we come across the various types and the inimitable features that make this synthetic all the more common.
Types and Characteristics of Polyester
Although it is not a hundred percent homogenous, it consists of different varieties with different properties and uses. One of the most known varieties is polyethylene terephthalate. It is prevalent in using it in clothing as well as manufacturing of plastic bottles. Another is polybutylene terephthalate, used in electronic parts because of its good electrical insulating properties. Other examples that are of specialized polyesters are elastomeric polyesters providing excellent stretch and recovery, hence used in the making of activewear and swimwear.
One of the biggest factors working in favor of polyester is the angle towards sustainability that comes along since many of these forms are in a recycled condition and originally came from plastic bottles that have already been used by people and then disposed of in landfills. It's proved to be a way by which the use of brands adopting recently replicated polyester expects to reduce the ecologic impact, and that holding a high value of quality. Each form of polyester has its purpose, and understanding the variation within the fabric is important for industries ranging from fashion to manufacturing. So its variability can be, in turn, adjusted to suit any application that is deemed fit, thus making it all the more relevant across a wide swathe of fields.
These are the polycondensation products of dicarboxylic acids and diols. Dacron or terylene is the best-known example of polyester. It is manufactured by heating a mixture of ethylene glycol and terephthalic acid at 420 to 460 K in the presence of zinc acetate-antimony trioxide catalyst as per the reaction given earlier. Dacron fiber (terylene) is crease-resistant and is used in blending with cotton and wool fibers and also as glass reinforcing materials in safety helmets, etc.
Relevance and Real-life Application
Polyester is not used only in textiles. In the fashion world, it has gained stellar reviews for being a material mimicking natural fibers, but which performs even better in attributes like endurance and low need for care. Thus, it has become one of the staples found in casual wear, sportswear, and uniforms. For example, manufacturers producing sportswear for athletes use it to a good extent as moisture-wicking clothing to keep the body dry and comfortable during workouts.
Besides clothing, polyester finds wide application in home furnishing. Polyester upholstery fabric is resistant to stains and does not wear off that easily or fade with rubbing. Far more importantly, it goes into the manufacture of insulating material, automotive fittings, and even medical appliances. Moreover, new ways are being researched for recycling polyester, which will help to further abate wastes while increasing the goal of sustainability. In the presented case studies, the companies integrated recycled polyester into their products. These opened the potential to bring about environmental benefits, especially since consumers today are more interested in 'green' products. Accordingly, as awareness of the environment increases, polyester plays a bigger role in sustainability.
Recommended topic video on(Polyester)
Some Solved Examples
Example 1
Question: Which one is classified as a condensation polymer?
1) Dacron
2) Neoprene
3) Teflon
4) Acrylonitrile
Solution: Dacron is classified as a condensation polymer, specifically a polyester formed from ethylene glycol and terephthalic acid. Neoprene, on the other hand, is an additional polymer made from chloroprene. Thus, the correct answer is option (1).
Example 2
Question: What are the monomer units of Dacron polymer?
1) Glycerol and phthalic acid
2) Ethylene glycol and phthalic acid
3) Ethylene glycol and terephthalic acid
4) Glycerol and terephthalic acid
Solution: The monomer units of Dacron, also known as Terylene, are ethylene glycol and terephthalic acid. Therefore, the correct answer is option (3).
Example 3
Question: Which of the following is an example of polyester?
1) Butadiene-styrene copolymer
2) Melamine polymer
3) Neoprene
4) Poly-β-hydroxybutyrate-co-β-hydroxyvalerate
Solution: Poly-β-hydroxybutyrate-co-β-hydroxyvalerate is an example of a polyester. Thus, the correct answer is option (4).
Example 4
Question: Given the statements:
Assertion A: Dacron is an example of a polyester polymer.
Reason R: Dacron is made up of ethylene glycol and terephthalic acid monomers.
Choose the most appropriate answer.
1) Both A and R are correct and R is the correct explanation of A.
2) Both A and R are correct but R is NOT the correct explanation of A.
3) A is correct but R is not correct.
4) A is not correct but R is correct.
Solution: Both the assertion and reason are correct, and the reason correctly explains the assertion. Therefore, the correct answer is option (1).
Summary
What has become a part of modern life is synthetic fiber—specifically, polyester. From being a polymer in definition to the kinds, especially PET and recycled polyester, this is a versatile, long-lasting material in its application in fashion, home furnishing, and industries—only mentioning some everyday uses and some academic research. Both during its making and through its use, polyester stands among the materials that lie at the heart of all contemporary discussions of a world poised uneasily on the precipice of the future.
