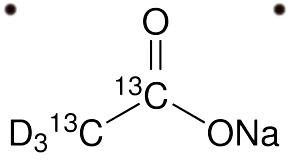
Sodium Acetate (CH3COONa) - Structure, Uses with FAQs
Sodium acetate, also denoted as NaCH3COO is the Na salt of acetic acid, which is colorless in appearance. The melting point of sodium acetate crystal is 324oC. when they are kept heating even after the physical constant and cooled down right after heating, the sodium acetate solution becomes saturated to its fullest. The molecular weight or the molar mass of sodium acetate is 82.03. The Ph of aqueous solution of sodium acetate is actually greater than 7 because on hydrolysis sodium acetate gives a combination of a weak acid and a strong base, this is the reason behind alkaline nature of aqueous solution of sodium acetate.
This Story also Contains
- Sodium acetic acid
- The structure of sodium acetate is given below
- Sodium acetate uses:
Sodium acetic acid
When sodium hydroxide is treated with acetic acid, sodium salt of acetic acid is formed.
NaOH + CH3COOH →CH3COONa + H2O
Sodium acetate anhydrous
Sodium acetate anhydrous is the Na salt form of acetic acid. Sodium acetate in anhydrous condition dissociates in water to form acetate ions and sodium ions. Sodium is the important cation of the cellular fluid outside the matrix and plays a big part in fluid and electrolytes replacement procedures.
Sodium acetate trihydrate
Sodium acetate trihydrate, is a trihydrate form of sodium salt of acid, which is used as a good source of sodium ions in aqueous solutions for dialysis and as a systematic and diuretic etc.
The molecular mass of sodium acetate trihydrate is 136.08.
Also read -
The structure of sodium acetate is given below
Sodium acetate uses:
- It is used in textile industries in corporation with aniline dye.
- It is used as a sealant in concrete used on construction sites.
- It is used as a buffer while performing lab practical in usage with acetic acid to keep constant P kb values in Ph dependent experiments.
- It is used in hot ice, thermal pads, and winter thermal wears.
- In chrome tanning procedures, sodium acetate is used as a pickling agent.
- While performing dialysis procedures, it is used a good source of sodium ions in aqueous solutions.
- In food industry, it is used as an additive and seasoning to give flavor and also used in preservation to get rid of food spoilage.
- It is also used to get of rid of accumulated static energy, in electrochemical operations.
- In the study of biotechnology, sodium acetate is largely used as carbon source provided in the nutrient medium for the growth and culture of bacterial analysis.
Sodium acetate in water
Sodium acetate gets easily soluble in water. The solubility of sodium acetate increases with the rise in temperature. The trihydrate form of sodium acetate is not as soluble in water as the anhydrous form of sodium acetate is.
The symbol of sodium acetate is CH3COONa.
Pharmaceutical uses of sodium acetate
- At pharmaceutical level, sodium acetate is a good component of electrolyte recharger which is given intravenously to the patient with low electrolytes level.
- It is also used in patients with hyponatremic illness to correct, incorrect sodium levels
- It is also used as urine alkalization.
Methane from sodium acetate
Sodium acetate when treated with calcium oxide upon heating, it gets converted to methane.
CH3COONa + NaOH → CH4 + Na2CO3
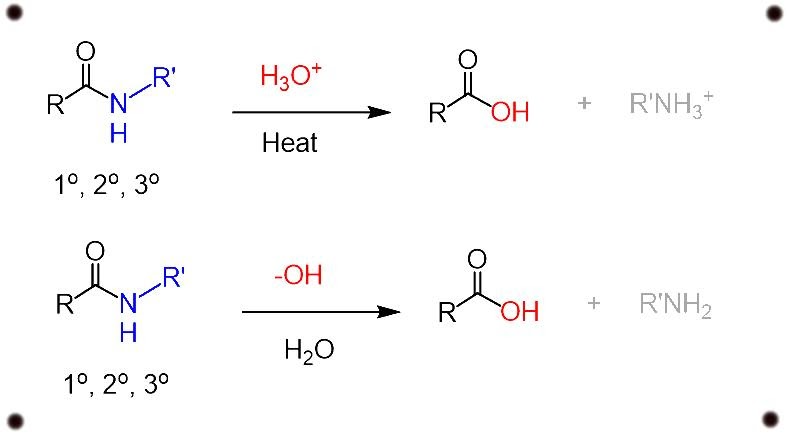
Amide hydrolysis mechanism
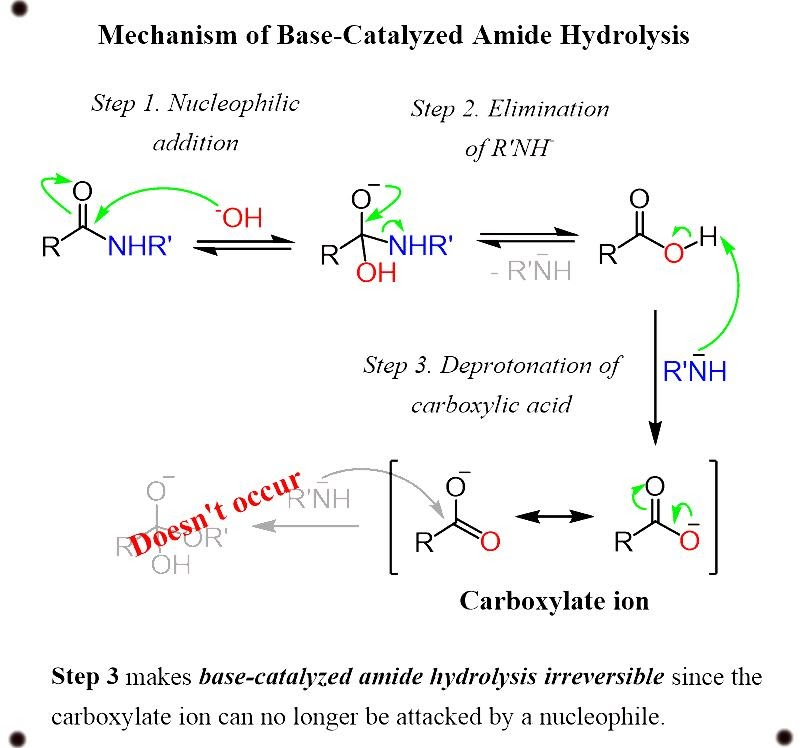
Base catalyzed hydrolysis of amides
Amides are the minimal reactive components of carboxylic acid because the conjugate base of an amine which is formed in a nucleophilic substitution reaction is a bad leaving group which contributes to the property of strong base.
For example, in the reaction with a hydroxyl group -OH, we are co-relating the leaving group property of the hydroxyl ion with the conjugate base of the ion.
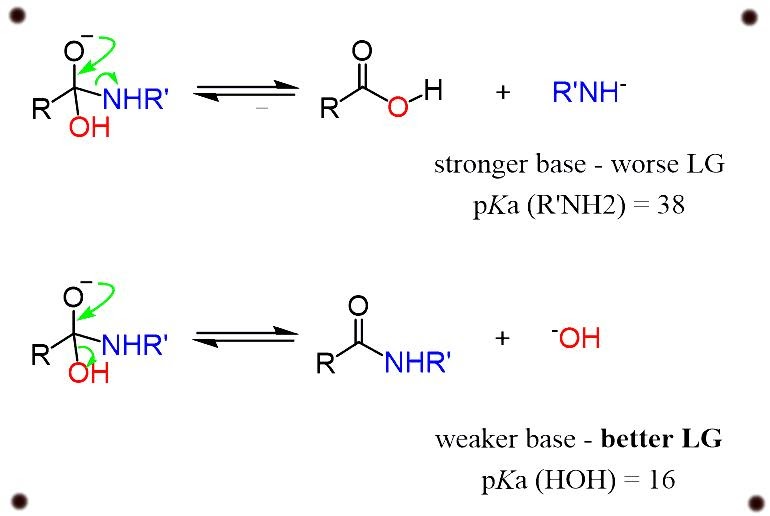
differentiating the P ka values of water and amines, we get to know that the hydroxyl groups are very much weaker bases and therefore are much better leaving groups.
| Related topics, |
Thus, the reaction does not exist to work.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 10 The S-Block Elements
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10 The S-Block Elements
- NCERT notes Class 12 Chemistry Chapter 10 The S-Block Elements
Acid catalyzed hydrolysis of amides
The acid catalyzed hydrolysis of amides have the same step as we have seen in ester hydrolysis, with the difference of the leaving group from ROH to RNH3+.
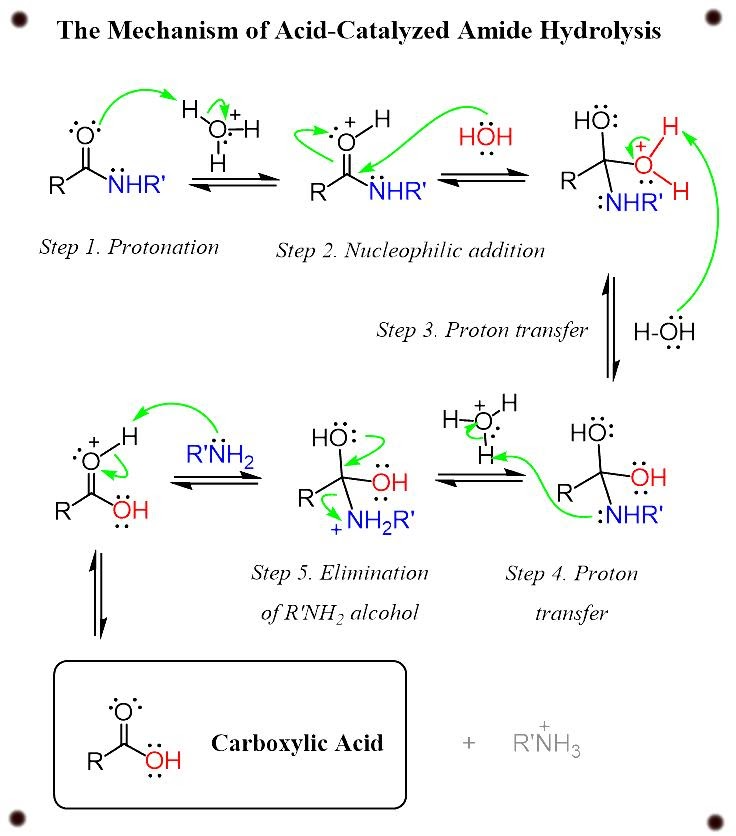
In all the above two cases, enthalpy and large number of extra reactants is needed to push the reactants forward in direction to complete the reaction.
Aqueous alkali
Alkalis as you know, are all Arrhenius bases, Arrhenius bases are the bases which form hydroxyl ions, upon dissolving in water.
Common properties of aqueous alkali include:
- Average concentrated solutions have a Ph of 7.1 or greater, which gives the understanding behind turning of phenolphthalein from colorless to pink.
Sodium ethanoate
Sodium ethanoate also abbreviated as Na2C2H3O2 is a colorless crystals which are soluble in water and has a melting point of 324oC, which is used in applications of organic synthesis as an chemical intermediate, in pharmaceutical and dye industries as well.
Alkyl ester
Ester is a chemical compound made out of an acid in which one hydroxyl group is replaced by an alkyl group -O- alkoxy group, as happened in the substitution reaction of alcohol and a carboxylic acid.
Alkyl esters are known as the softest ester of all applied in hair care and body care products. People use it as a silicone restoring procedures, it reduces the gaudy feeling to almost any emulsion, it is also is non-comedogenic, and can also be used as a skin conditioner in oil free based products.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: