Tautomerism
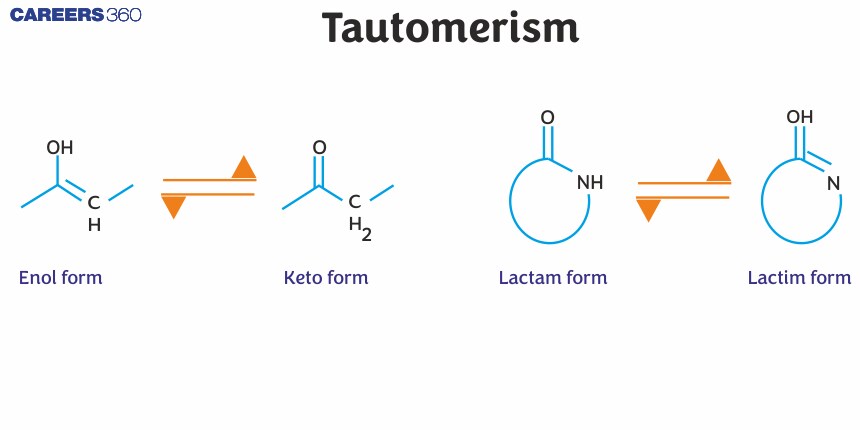
Tautomerism is a phenomenon where a single chemical compound tends to exist in two or more interconvertible structures that are different in terms of the relative position of one atomic nucleus, which is generally hydrogen. When a reaction occurs between these compounds, there is only a transfer of protons. Tautomerism is also termed as desmotropism. The two structures are called tautomers, and these types of isomer compounds usually differ only in the number of electrons and protons.
This Story also Contains
- Tautomers
- Solved Examples Based on Tautomerism
- Conclusion
In this article, we will cover the topic of tautomerism). This topic falls under the broader category of (Some Basic Principles of Organic Chemistry), which is a crucial chapter in (Class 11 Chemistry). It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more
Tautomers
Tautomers are isomers of a compound that differ only in the position of H+ and electrons. The carbon skeleton of the compound is unchanged. A reaction that involves simple proton transfer in an intramolecular fashion is called tautomerism.

In the above molecule, the $\alpha-\mathrm{H}$ has changed its position from carbon to oxygen atom with the rearrangement of the double bond. This can be visualized in the following manner hypothetically:
(1) Breaking of $\mathrm{C}-\mathrm{H}$bond and generation of Carbanion at $\alpha-C$
(2) Resonance of the carbanion with the conjugated keto group
(3) Attachment of the proton at the O atom carrying a negative charge
Can you write the above steps in a diagrammatic fashion to get a better understanding?
It is to be noted that the keto form is preferred over the enol form in most of the cases. This is because of the high bond energy of the $\mathrm{C}=\mathrm{O}$. However, the enol form is more stable under cases where the product is aromatic (Phenol) or in the case of 1,3- dicarbonyl compounds which are stabilized due to intramolecular H-Bonding.
 : Aromaticity
: Aromaticity

Intramolecular H Bonding
Some other cases where Tautomerism is seen
- Enamine -Imine
$\mathrm{NH}_2-\mathrm{CH}=\mathrm{CH}_2 \rightleftharpoons \mathrm{NH}=\mathrm{CH}-\mathrm{CH}$
- Nitroso - oxime
$\mathrm{CH}_3-\mathrm{N}=\mathrm{O} \rightleftharpoons \mathrm{CH}_2=\mathrm{N}-\mathrm{O}-\mathrm{H}$
Recommended topic video on (Tautomerism)
Solved Examples Based on Tautomerism
Q.1 How many tautomers can you draw for the following ketone?

(1) 1
(2) 2
(3) 3
(4) 4
Solution:
As we have learned
Definition of Tautomerism -
Tautomers are isomers of a compound that differ only in the position of the protons and electrons. The carbon skeleton of the compound is unchanged. A reaction that involves simple proton transfer in an intramolecular fashion is called tautomerism.

There are 2 possible.
Therefore, option (2) is correct.
Q.2 Identify the compound that exhibits tautomerism.
(1) 2-Butene
(2) Lactic acid
(3) 2-Pentanone
(4) Phenol
Solution:
As we have learned
2- pentanone shows tautomerism due to the presence of alpha hydrogen near the $\mathrm{C}=\mathrm{O}$ group.

Therefore, option (3) is correct.
Conclusion
We now know that tautomers are the isomers of a compound that differ only in the position of the protons and electrons. A reaction that involves simple proton transfer in an intramolecular fashion is called tautomerism. The structural Requirement of Tautomerism has also been discussed. Different types of tautomerism and Tautomerism reaction mechanisms have been talked about in detail. We have gone through propyne and allenes. The Allenes are organic compounds in which one carbon atom has a double bond with each of its two adjacent carbon centers.