Aluminium Oxide (Al2O3): Structure, Properties, Production, Uses
Al_{2}O_{3}![]() is the chemical formula for the compound of Aluminium and Oxygen known as Aluminium Oxide which is also called alumina. Depending on specific forms or uses, it may also go by the names Aloxide, Aloxite, or Alundum. It naturally occurs as Corundum (\alpha-Al_{2}O_{3}
is the chemical formula for the compound of Aluminium and Oxygen known as Aluminium Oxide which is also called alumina. Depending on specific forms or uses, it may also go by the names Aloxide, Aloxite, or Alundum. It naturally occurs as Corundum (\alpha-Al_{2}O_{3}![]() ), a mineral that gives rise to the valuable gemstones Ruby and Sapphire, in its crystalline polymorphic phase. Al_{2}O_{3}
), a mineral that gives rise to the valuable gemstones Ruby and Sapphire, in its crystalline polymorphic phase. Al_{2}O_{3}![]() plays an important role in the production of Aluminium metal, as an abrasive due to its hardness, and as a refractory material due to its high melting point.
plays an important role in the production of Aluminium metal, as an abrasive due to its hardness, and as a refractory material due to its high melting point.
This Story also Contains
- Natural Occurrence Of Aluminium Oxide
- Physical Properties Of Aluminium Oxide
- Chemical Properties Of Aluminium Oxide
- Production Of Aluminium Oxide
- Uses Of Aluminium Oxide
.png)
Natural Occurrence Of Aluminium Oxide
The most prevalent naturally occurring crystalline form of Aluminium Oxide is Corundum. The gem-quality Corundum varieties known as Rubies and Sapphires receive their distinctive colours from trace impurities. Traces of Chromium give rubies their distinctive deep red colour and their laser-like properties. Sapphires have a variety of colours that are caused by several additional impurities, including Iron and Titanium. The mineral Deltalumite is a δ-form that is relatively uncommon.
Physical Properties Of Aluminium Oxide
Aluminium Oxide is an odourless white crystalline powder which is insoluble in water. Aluminium Oxide has insulating properties and very high thermal conductivity.
Chemical formula of Aluminium Oxide = Al_{2}O_{3}
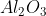 .
.The Colour of Aluminium Oxide = White.
Colour of the most common form of Aluminium Oxide (corundum) - Rubies are red and sapphires are blue, black, pink etc.
Molecular/Molar mass of Aluminium Oxide = 101.96 g/mol
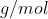 .
.Density of Aluminium Oxide = 3.987 g/cm^{3}
 .
.The melting point of Aluminium Oxide = 2,072 ℃.
The boiling point of Aluminium Oxide = 2,977 ℃.
Chemical Properties Of Aluminium Oxide
The reaction of Aluminium Oxide with Sodium Hydroxide.
Sodium Aluminate and water is formed when Aluminium Oxide reacts with Sodium Hydroxide. The temperature where this reaction occurs is between 900 ℃ and 1100 °C. In this reaction, Aluminium Oxide functions as an acid to produce salt and water.
Al_{2}O_{3}+2NaOH\rightarrow 2NaAlO_{2}+H_{2}O
![]()
The reaction of Aluminium Oxide with Sulphuric Acid.
Although most metal oxides are basic, Aluminium Oxide is an amphoteric oxide. So it functions as both an acid and a base. It acts as a base in this instance and a neutralization reaction occurs to produce salt and water.
Al_{2}O_{3}+H_{2}SO_{4}\rightarrow Al_{2}\left ( SO_{4} \right )_{3}+H_{2}O
![]()
The reaction of Aluminium Oxide with Hydrochloric Acid.
Since Aluminium Oxide includes oxide ions, it reacts with acids like the way Sodium or Magnesium oxides react. An Aluminium Chloride solution is produced when Aluminium Oxide and hot, diluted Hydrochloric Acid react.
Al_{2}O_{3}+6HCl\rightarrow AlCl_{3}+3H_{2}O
![]()
Production Of Aluminium Oxide
Bauxite, the primary ore of Aluminium, mostly consists of Aluminium Hydroxide. The minerals that make up Bauxite ore also include Gibbsite (Al\left ( OH \right )_{3}![]() ), Boehmite (\gamma-AlO\left ( OH \right )
), Boehmite (\gamma-AlO\left ( OH \right )![]() ), and Diaspore (\alpha-AlO\left ( OH \right )
), and Diaspore (\alpha-AlO\left ( OH \right )![]() ), as well as Clay, Quartz, and Iron Oxide and Hydroxide impurities. Usually, the Bayer process is used to purify the Bauxite ore:
), as well as Clay, Quartz, and Iron Oxide and Hydroxide impurities. Usually, the Bayer process is used to purify the Bauxite ore:
Al_{2}O_{3}+H_{2}O+NaOH\rightarrow NaAl\left ( OH \right )_{4}
![]()
Al(OH)_{3}+NaOH\rightarrow NaAl(OH)_{4}
![]()
The remaining components of Bauxite, besides Silicates SiO_{2}![]() , do not dissolve in the base. Fe_{2}O_{3}
, do not dissolve in the base. Fe_{2}O_{3} ![]() is eliminated after filtering the basic mixture. Al(OH)_{3}
is eliminated after filtering the basic mixture. Al(OH)_{3}![]() precipitates when the Bayer liquid cools, leaving the Silicates in solution.
precipitates when the Bayer liquid cools, leaving the Silicates in solution.
NaAl(OH)_{4}\rightarrow NaOH+Al(OH)_{3}
![]()
Aluminum Oxide is produced by Calcining (heating to a temperature of above 1100 °C) the solid Al(OH)_{3}![]() -Gibbsite.
-Gibbsite.
2Al(OH)_{3}\rightarrow Al_{2}O_{3}+3H_{2}O
![]()
In contrast to Corundum, the final product of Aluminium Oxide produced by Bayer's process often consists of many phases of Aluminium Oxide. As a result, the manufacturing procedure may be improved to provide a customised product. The different phases that are present, for instance, have an impact on the solubility and pore structure of the Aluminium Oxide product, which has an impact on the price of producing Aluminium and control of pollution.
Uses Of Aluminium Oxide
Aluminium Oxide, which is white and mostly chemically inert, is a preferred filler for plastics. Sunscreen frequently contains the mineral Aluminium Oxide, which is also occasionally found in cosmetics like blush, lipstick, and nail paint.
Aluminium Oxide is widely used in ceramic industries.
Aluminium Oxide is a common component in glass formulations. Commonly used Aluminosilicate glass frequently has 5% to 10% alumina in it.
Several relevant industrial processes are catalysed by Aluminium Oxide. In its most extensive use, Aluminium Oxide serves as the catalyst in the Claus process, which refineries use to convert Hydrogen Sulphide waste gases into elemental Sulphur. Additionally, it helps dehydrate Alcohols into Alkenes. For many industrial catalysts, including those used in Hydrodesulfurization and several Ziegler-Natta polymerizations, Aluminium Oxide acts as a catalytic support.
Water removal from gas streams is frequently accomplished using Aluminium Oxide.
Aluminum Oxide is utilised for its strength and hardness. On the Mohs scale of mineral hardness, its naturally occurring form, Corundum, rates a 9 (just below diamond). It is frequently used as an abrasive, especially as an industrial Diamond alternative since it is so much less costly. Aluminium Oxide crystals are used in several types of sandpaper. Additionally, due to its low specific heat and low heat retention, it is frequently utilised in grinding operations, notably for cutoff tools.
For reflected aesthetic effects, such as in the automotive or cosmetic sectors, Aluminium Oxide flakes are employed in paint.
Alumina ceramic plates are sometimes used in body armour, typically in conjunction with aramid or UHMWPE backing to obtain efficacy against the majority of rifle threats.
Anodizing or plasma electrolytic oxidation can be used to generate Aluminium Oxide as a coating on Aluminium. The high strength of Aluminium Oxide is the source of the coating's hardness and abrasion resistance.
In coal-fired power plants, Alumina is used to make the tiles that are installed inside the pulverised fuel lines and flue gas ducting to cover high-wear regions.
In addition to serving as a tunnel barrier for the construction of superconducting devices like single-electron transistors and superconducting quantum interference (SQUIDs) devices, the electrical insulator Aluminium Oxide is also employed as a substrate for integrated circuits.
Frequently Asked Questions (FAQs)
Aluminium Oxide is used in industries as
Abrasive,
Catalyst,
Filler,
Heat stabilizers,
Insulators,
Adsorbents etc.
By inhaling its aerosol, ingesting it, coming into contact with the skin or eyes, or consuming it, Aluminium Oxide can be absorbed into the body. It could irritate your eyes, skin, or respiratory system.
Most metal oxides are basic but Aluminium Oxide is amphoteric in nature hence it is both acidic and basic in nature.
An inert mixture of Aluminium and Oxygen is known as Aluminium Oxide. Al2O3, a non-metal or pure ceramic, is created by the oxidation of Aluminium and Oxygen.
Aluminium metal quickly forms a few millimetre-thick coating of Aluminium Oxide that stops it from interacting with water. This layer corrodes, causing a reaction that releases extremely flammable hydrogen gas.
