Components of Air - Oxygen, Nitrogen, Carbon Dioxide, Water with FAQs
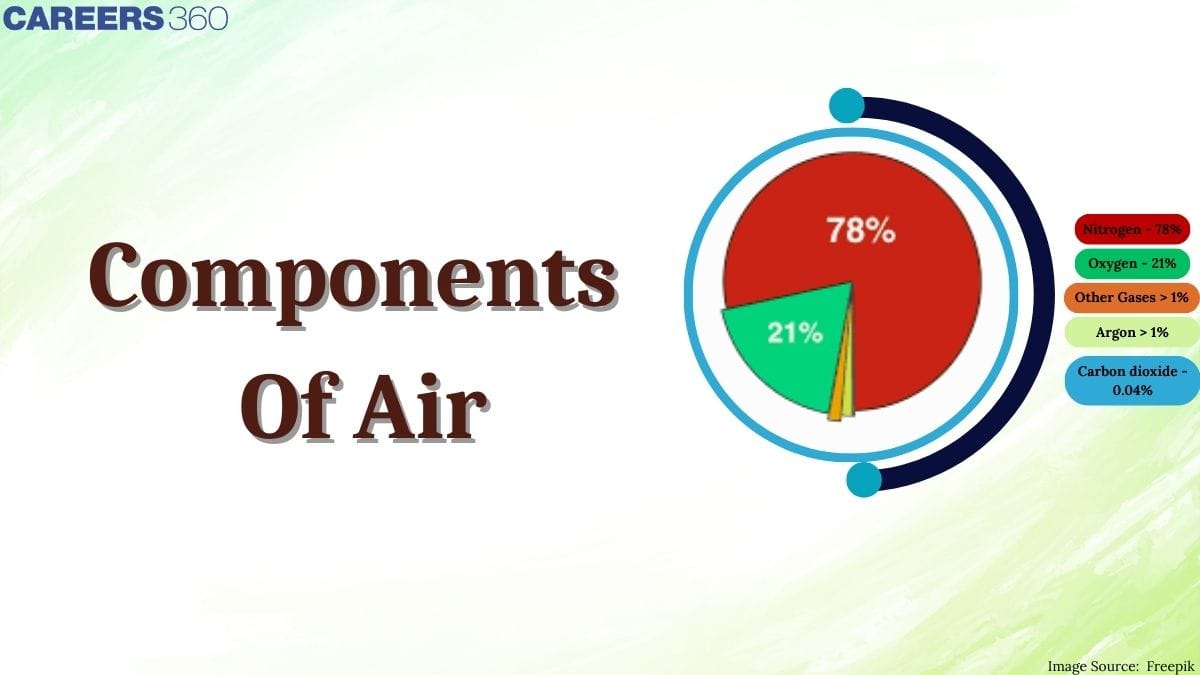
Have you ever thought about what exactly makes up the air we breathe every second? Why is nitrogen, which forms about 78% of air, so important even though we cannot directly use it for respiration? How does oxygen, nearly 21% of air, become the lifeline for animals and the driving force behind combustion? You will get all these answers after reading this article on component of air. Air contains a mixture of gases that surrounds the Earth and is essential for life. Its composition (by volume) in the lower atmosphere is approximately 78% nitrogen, 21% oxygen, 0.93% argon and 0.03–0.04% carbondioxide and many other gases.
This Story also Contains
- Air
- Constituents of Air
- Oxygen
- Carbondioxide
- Nitrogen
- Hydrogen
Air
- Air that we breathe in is available anytime and anywhere around us. Air cannot be seen as the other necessities of life such as water, sun, and food.
- Dip an empty bottle in a large container of water. You will observe bubbles evolving out of it. It means you are replacing the air present in the bottle with water without seeing it. However, the air is an indispensable part of our lives. Let us see what makes air a crucial part of life and what are the constituents of air.
- Air is a mixture of gases that is a part of the atmosphere. The major components of the atmosphere are substances such as air, water vapors, and dust particles.
- The major component of air is composed of nitrogen and oxygen alone.
- Until the 15th century, the air was considered to be an element. Not long after, Lavoisier became the first scientist to recognize that the ratio of nitrogen to oxygen in the air is 4:1.
- A Large Percentage of air in the atmosphere has nitrogen and oxygen as the major components. The atmosphere protects us from hazardous radiations of the space and extends up to a height of 20 km and above. There is a Huge Difference between air and oxygen that we will see in a while.
Also read -
Constituents of Air
Air contains a mixture of gases. A considerable segment of the air is constituted by oxygen and Nitrogen. The major component of air is composed of active and inactive components.
However, this mixture of many gases has the highest percentage of nitrogen in atmosphere which is an inactive component. The percentage of nitrogen in air is 78% and O2 percentage in the air is 21%.
Nitrogen is the inactive part of the atmosphere because it cannot be directly used by us and oxygen in the air is the active part of the atmosphere because it supports life.
Air consists of many harmful particles like smoke and dust particles. Smoke consists of nanosized carbon particles that remain unburnt during the burning of a fuel.
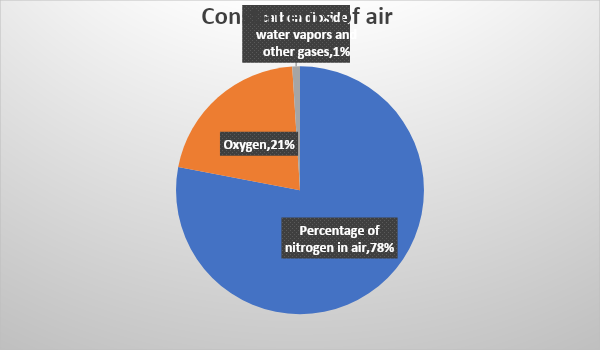
Constituents of Air Diagram
Above is a pie chart showing the composition of air and present gases in the atmosphere and their percentages.
The various constituents of air and their uses are given below-
Oxygen
- Carl Scheele was the first scientist to witness combustion supporting the nature of oxygen in the air. Names like “vital air” and “fire air” were given to oxygen.
- Oxygen was prepared in many ways like by decomposition of mercuric oxide and by focusing sun rays through the convex lens on mercuric oxide.
- Later Antoine Lavoisier proved oxygen to be an element. The name ‘Oxygen’ was given by Lavoisier after he found oxygen helpful in the production of acid.
- Free state- Atmospheric air consists of oxygen in its elemental state as 21% by volume and 23% by mass.
- Combined state- the percentage of oxygen in water is a tiny amount as compared to the percentage present in air i.e.,1%. This oxygen exists in water in a combined state and is the source of survival for aquatic life.
Chemical Properties of oxygen
- The component of air that supports combustion is oxygen. The volume of oxygen in air supports combustion however it doesn't burn on its own. Hence, it’s a non-combustible gas.
- Oxygen supports rusting in which iron gets converted to rust (a hydrated form of iron).
- It is neutral (neither acidic nor basic,)
Uses of Oxygen-
- Respiration- Living beings inhale oxygen and ultimately produce energy.
- Used in carbogen (a mixture of 95% oxygen and 5% carbon dioxide) given to patients with breathing problems.
- Oxygen is essential for the ignition of fuels.
- Cartridge- A mixture of liquid oxygen, liquid petroleum, and coal is used for blasting mountains.
Carbondioxide
- Carbon dioxide is the fifth abundant gas in the atmosphere. It occurs in 0.003% of the total volume of air.
- It is an odorless gas that is highly soluble.
- It is heavier than air and is acidic.
- Carbon dioxide occurs if free as well as in a combined state. In combined forms, it exists as carbonates in limestone, dolomite, etc. It is present in its free state in water bodies like lakes, oceans, etc.
- Carbon dioxide was first called Carbonium by van Helmont and later it was called acid carbonium by Lavoisier.
- Joseph Black synthesized the gas by heating magnesium carbonate.
| Related topics link, |
Chemical Properties of Carbon dioxide-
- It has no combustibility properties as it is not a combustible gas and cannot support combustion.
- When CO2 reacts with water it becomes acidic. The compound formed is known as carbonic acid.
- Carbon dioxide is produced when coal and natural gas are formed.
- Carbon dioxide reacts with metals and non-metals to form metal carbonates and metal oxides respectively.
Uses of Carbon dioxide-
- It is an important compound for the formation of sodium hydrogen carbonate, washing soda, etc.
- The tangy taste of soft drinks is imparted due to dissolved carbon dioxide in liquids.
- It is used for the preservation of food items.
- CO2 present in the atmosphere is taken up by the plants through photosynthesis to produce oxygen.
- Dry ice is used in refrigerators to store fruits and vegetables.
Also Read:
Nitrogen
The percentage of nitrogen in air is 78% by volume Main component of air is nitrogen gas.
- Like carbon dioxide and oxygen, nitrogen also exists in combined and free states. Examples- KNO3,NaNO3, etc.
- Nitrogen in the air is also an inactive component of the atmosphere since it weakens the activity of oxygen and slows down the rapid burning.
Chemical properties of Nitrogen-
- Nitrogen fixation- Elemental state of nitrogen in the air is present in the atmosphere in a free state but cannot be directly used therefore, it is first fixed by leguminous plants by converting nitrogen into essential compounds of nitrogen. This process is called nitrogen fixation which is very essential to make use of the nitrogen present in the atmosphere.
- Leguminous plants have symbiotic bacteria in their root nodules which assimilate nitrogen present in the atmosphere and convert it into essential nitrogen compounds.
Uses of nitrogen-
- Nitrogen is used for the conservation of food items since it is chemically inert.
- Nitrogen is essential for the synthesis of proteins.
- Nitrogen (i.e., a major component of air) slows down the activity of oxygen.
Hydrogen
- Hydrogen is mostly found in the combined state in various carbon compounds such as vitamins, nucleic acids, enzymes, etc.
- Non-living matter such as wood, paper, and rubber contains hydrogen.
- Henry Cavendish was the first person to establish the elemental nature of hydrogen.
- The first method to produce hydrogen was from the action of sulfuric acid on metals.
- The name hydrogen means water producing. Hydro means water and gen means to produce.
- Hydrogen is the lightest element that is present in trace amounts in the atmosphere.
Chemical properties of hydrogen-
- Hydrogen storage is a major issue since it reacts with oxygen and burns in the air.
- Hydrogen reacts with non-metals to form metal hydrides.
- Hydrogen reacts with nitrogen in the ratio of 3:1 to form ammonia.
- Hydrogen adds to many unsaturated molecules to form saturated molecules. This is known as hydrogenation.
Uses of hydrogen-
- Hydrogen is essential in Haber’s process for the formation of ammonia.
- Many other important compounds such as hydrogen chloride and methyl alcohol are synthesized with the help of hydrogen.
- Hydrogen is used to extract some metals from their oxides.
- Hydrogen can react with compounds in the form of nascent hydrogen [H]. This nascent hydrogen is called newly born hydrogen. This is important for the compounds which do not react with hydrogen originally.
Also check-
Some Solved Examples
Question 1: Which is the major constituent of air by volume?
a) Oxygen
b) Carbon dioxide
c) Nitrogen
d) Argon
Solution: Dry air contains ~78% nitrogen, ~21% oxygen, ~1% argon, and traces of others.
Hence, the correct answer is option is (c)
Question 2: What is the approximate percentage of carbon dioxide in the atmosphere?
a) 21%
b) 0.03–0.04%
c) 78%
d) 1%
Solution: $\mathrm{CO}_2$ is a minor component but plays a vital role in photosynthesis and greenhouse effect.
Hence, the correct answer is option (b)
Question 3: Which gas in air supports burning and respiration?
a) Nitrogen
b) Oxygen
c) Carbon dioxide
d) Argon
Solution: Oxygen (21%) is essential for respiration and combustion.
Hence, the correct answer is option is (b)
Frequently Asked Questions (FAQs)
The least percentage of air is 1% which is composed of particles and gases such as inert gases, carbon dioxide, hydrogen, smoke, and dust particles.
78% of the air by volume percentage is composed of nitrogen gas.
Nitrogen content in the air is the highest. Although this nitrogen present cannot be directly used. This nitrogen is first converted by the leguminous plants in their root nodules to synthesize nitrogen compounds. This process is called nitrogen fixation.
A major component of air is Nitrogen gas (78%). It is an inactive component of the air and slows down the activity of oxygen.
Air is mainly composed of nitrogen (78%), oxygen (21%), trace gases (1%) such as inert gases, water vapors, smoke, and dust particles.
Nitrogen and oxygen together make the major constituent of air (78%+21%) =99%
