Aliphatic Hydrocarbons - Definition, Examples, Properties, FAQs
Aliphatic compounds definition
Hydrocarbons are compounds that contains carbon and hydrogen. Hydrocarbons are classified as aromatic compounds and aliphatic compounds. In organic chemistry an aliphatic compound or aliphatic meaning is compounds containing hydrogen and carbon atoms that are usually combined together in straight chains but sometimes the chains are also in the form of non-aromatic structures.
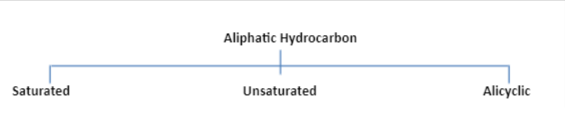
Aliphatic compounds can be saturated or unsaturated. Straight or branched open-chain compounds, which involve no rings of any type, are aliphatic. If cyclic compounds are not aromatic they are aliphatic.
Difference between aliphatic and aromatic hydrocarbons
Compounds formed of mainly two elements carbon and hydrogen bonded to each other through covalent bonding are known as Hydrocarbons. Depending on the arrangement of atoms they are classified into two aliphatic hydrocarbons and aromatic hydrocarbons. In organic chemistry hydrocarbons formed of a carbon atoms and a hydrogen atoms present in a straight chain or branched form are aliphatic hydrocarbons.
Compounds that are composed of carbon atoms and hydrogen atoms in ring structures having delocalized pi electrons are aromatic hydrocarbons. Carbon-to-hydrogen ratio of aliphatic hydrocarbons are high but carbon-to-hydrogen ratio of aromatic hydrocarbons are low. This is the main difference between aliphatic and aromatic compounds.
Aromatic compounds have pleasant odour but aliphatic compounds do not have pleasant odour. All aromatic hydrocarbons are unsaturated but all aliphatic hydrocarbons are not unsaturated, some of them are saturated and others are unsaturated. The saturated meaning in Guajarati is santrpta.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Aliphatic acid
The acids of non-aromatic hydrocarbons are known as aliphatic acid. For example acetic, propionic, butyric acid; the so-called fatty acids with the formula R-COOH and R represent non-aromatic hydrocarbons or aliphatic hydrocarbons.
Aliphatic alcohol
Aliphatic alcohols are organic compounds. They are flammable liquids and have higher solubility in water and many other organic solvents. Highly volatile liquids, they have stability in water under typical use situations. Methyl alcohol, ethyl alcohol and isopropyl alcohol are examples of aliphatic alcohol. Aliphatic alcohols are used as hard surface treatment disinfectants, sanitizers, virucides and fungicides.
Ethanol is used as a plant growth regulator for example as a ripener, and is utilized with quaternary ammonium compounds in swimming pool water systems. Isopropanol is another aliphatic alcohol that is having application in combination with other pesticide active substances to kill household insects like fleas and ticks. Both aliphatic alcohol like ethanol and isopropanol are well known substances that have a wide range of human uses and applications.
Examples are, ethanol is used in some beverages, and isopropanol utilized as a major ingredient in rubbing alcohol. Aliphatic alcohols are also used as surface wipes, sprays, sponge-on and wipe-on or pour-on treatments by immersion and through closed systems for commercial or industrial water cooling systems.
Saturated and Unsaturated Hydrocarbons
Aliphatic organic compounds may be saturated, unsaturated and alicyclic in nature. Alkanes are saturated hydrocarbons that are open chain hydrocarbons containing single bond of carbon atoms. Mostly they exist on covalent bonds. These are inert compounds and do not readily react with any acid, bases or other reagents. Unsaturated compounds are hydrocarbon molecules having one double bond. It is possible to add more hydrogen atoms to these molecules because of the presence of double bonds. These types of molecules are more reactive than saturated compounds.

ALICYCLIC;
Saturated hydrocarbon molecules are hydrocarbon molecules which do not have double bonds in them. This means that it is not possible to add extra hydrogen atoms to the molecule. Alkenes and alkynes are unsaturated compounds. Alkenes have at least one carbon-carbon double bond and Alkynes have at least one carbon-carbon triple bond. Saturated hydrocarbons are less reactive compared to unsaturated hydrocarbons. Normally unsaturated have fewer hydrogen atoms present in bond with carbon atoms.
Alicyclic compounds are compounds that do not have aromatic character. Monocyclic cycloalkanes are aliphatic. Alicyclic compounds include all monocyclic cycloalkanes like cyclopropane, cyclohexane, cycloheptane etc and bicyclic alkanes (bicycloundecane, decalin, and housane), and polycyclic alkanes (cubane, basketane, and tetrahedrane). Two or more rings that are connected through only one carbon atom are spiro compounds. The cycloalkanes are monocyclic saturated hydrocarbons that contain hydrogen and carbon atoms only.
They are arranged in a single ring structure that contains single bonded carbon atoms. Cyclopropane, cyclobutane, cyclopentane, and cyclohexane are some examples of cycloalkanes. Cycloparaffins are larger cycloalkanes consisting of more than 20 carbon atoms. The cycloalkanes that do not contain any side chains are classified as small, common, medium and large.
Small-cyclopropane and cyclobutane
Common-cyclopentane, cyclohexane, and cycloheptane
Medium- cyclooctane through cyclotridecane
Large– (all other larger)
Properties of Aliphatic Hydrocarbons
- Most of the aliphatic compounds are flammable, and these hydrocarbons are used as fuel. For example methane in Bunsen burners, liquefied natural gas and acetylene in welding.
- Aliphatic compounds are cyclic or acyclic. They consist of close chains or rings of carbon atoms in their molecules.
- Boiling point and melting point – Bonding between carbon and hydrogen is very weakly polar because of the small difference between the electronegativities. Decrease in the area of contact happens due to the chain branching. Hence one with highly branched alkanes has a lower boiling point if two alkanes have the same molecular weight. In aliphatic hydrocarbons as size increases melting point also increases but in a less regular manner.
- Solubility – In water and other polar solvents hydrocarbons tend to be insoluble as they are non-polar. In non-polar solvents such as benzene and diethyl ether hydrocarbons prefer to dissolve. Hence hydrocarbons are hydrophobic or lipophilic.
- Density - The hydrocarbons float on the water surface because of lesser density.
Aliphatic compounds examples
Examples of aliphatic hydrocarbon compounds are n-, iso- and cyclo-alkanes are saturated hydrocarbons and n-, iso- and cyclo-alkenes and -alkynes are unsaturated hydrocarbons.
Number of Carbon atoms | Aliphatic Hydrocarbons |
1 | Methane (CH4) |
2 | Ethane (C2H6), ethene (C2H4) , ethyne (C2H2) |
3 | Propane (C3H8), propene (C3H6), propyne C3H4), cyclopropane (C3H6) |
4 | Methylpropane (C4H10), butane (C₄H₁0), cyclobutene (C4H6) |
5 | Cyclopentene (C5H8), pentane (C5H12), dimethylpropane (C5H12) |
6 | Hexane (C6H14), cyclohexene (C6H10), cyclohexane (C6H12) |
7 | Cycloheptane (C7H14), cycloheptene (C7H12), heptane (C7H16) |
8 | Octane (C₈H₁₈), cyclooctane (C8H16), cyclooctene (C8H14) |
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 13 Hydrocarbons
- NCERT notes Class 11 Chemistry Chapter 13 Hydrocarbons
Extraction of Aliphatic Hydrocarbons
Pressurized Fluid Extraction processes are used for extraction of aliphatic compounds. Organic and aqueous extraction solvents are used for this. To extract aliphatic hydrocarbons, water which is converted to hot steam also be used mainly for the conversion from solid and semi-solid. In conventional flame spectrometry, there has been only minimum utilization of aliphatic hydrocarbons as solvent. Periodically, they get utilized as diluents for other solvents.
In conventional flame spectrometry only little use has been made from aliphatic hydrocarbons as solvents. For the nickel determination in an oxygen cyanogen flame pentane, cyclohexane and methyl cyclopentane are tested as solvents. These solvents offer excellent sensitivity when pentane was utilized in AAS but it has no other striking advantages. Heptane, cyclohexane and cyclohexene are found solvents possible for the beryllium determination by AFS. Occasionally, aliphatic hydrocarbons are utilized as diluents for other solvents.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: