Aromatic Compounds - Definition, Examples, Features, Characteristics, FAQs
Have you ever wondered why certain organic compounds like benzene have such a distinct smell and more stability? Why are some compounds have a pleasant odor? These questions lead us to the fascinating world of aromatic compounds a special class of organic molecules that follow Hückel’s rule. Chemical compounds that consist of ring systems associated with pi-electrons separated by alternating double and single bonds are called aromatic compounds. In the article we will study about aromatic compound in detail with some solved examples.
This Story also Contains
- IUPAC Name of Aromatic Compounds
- Examples of Aromatic Compounds
- Characteristics of Aromatic Compounds
- Non-benzenoid Aromatic Compounds
- Some Solved Examples
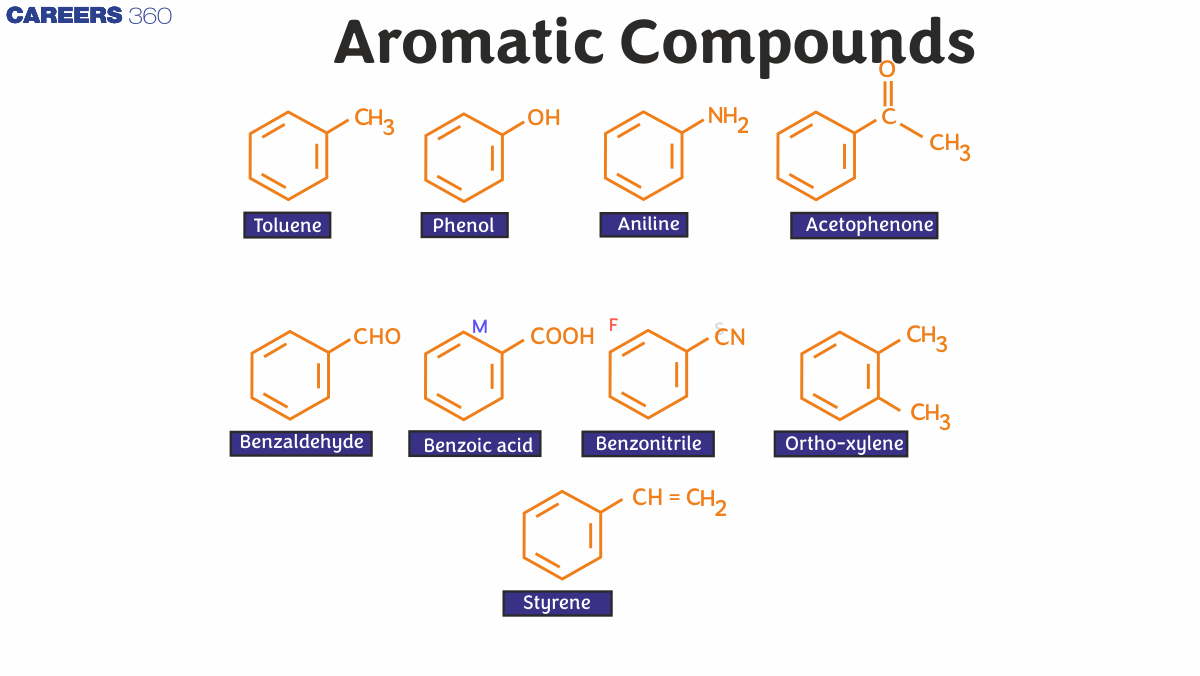
IUPAC Name of Aromatic Compounds
Previously, most chemicals with the same structure were known by different names depending on the regions in which they were synthesized. This naming system was relatively simple because it caused a great deal of confusion. Finally, a standard naming system that registered general rules was developed by IUPAC (International Union for Pure and Applied Chemistry) for compounds.
This method of naming is callecd IUPAC .
Some Rules of IUPAC for Aromatic Compound
1. Identify the Parent Compound
-
The basic structure is benzene $\left(\mathrm{C}_6 \mathrm{H}_6\right)$..
-
If the benzene ring is the main structure → name as a derivative of benzene.
-
If the benzene ring is a side group → it is called a phenyl group $\left(-\mathrm{C}_6 \mathrm{H}_5\right)$.
2. Monosubstituted Benzene
-
When only one substituent is attached, name it as a prefix before “benzene.”
Example: $\mathrm{CH}_3-\mathrm{C}_6 \mathrm{H}_5 \rightarrow$ Methylbenzene (Toluene)
3. Disubstituted Benzene
Use numbering or position prefixes to indicate where the substituents are attached.
-
1,2– or ortho (o–)
-
1,3– or meta (m–)
-
1,4– or para (p–)
Example: 1,3–Dinitrobenzene → m–Dinitrobenzene
4. Polysubstituted Benzene
-
Number the ring to give the lowest possible locants.
-
Start numbering from the substituent that gives the characteristic name (like –OH in phenol).
-
List substituents in alphabetical order.
Example: 1–Chloro–2,4–dinitrobenzene
5. Phenyl as Substituent
-
When benzene is attached to a longer carbon chain (more than 6 carbons), it acts as a phenyl group.
Example: $\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{CH}_3 \rightarrow 1$-Phenylethane
Examples of Aromatic Compounds
Fragrant hydrocarbons, with hydrocarbons containing sigma bonds and pi electrons separated between carbon atoms in the ring.
For example, benzene. They are known as aromatic because of their pleasant aroma.
Any hydrocarbon can be classified as an aromatic compound as long as it follows the Huckel rule. According to Huckel's law, for a ring to smell, it must have the following properties: The planet Complete the electron renewal in the ring
Presence of (4n + 2) π electrons in the ring when n is the sum (n = 0, 1, 2, ..)
Huckel's Law of Aromaticity
- Huckel's law states that only planets, fully monocyclic planets, have 4n + 2 electrons, where n is a whole number, i.e., n = 0, 1, 2, 3, 4, etc., which must have fragrant stability.
- The fragrant compound must be planetary and contain a circular cloud of electrons below and above the plane of the molecule.
- It has sp2 atoms separated and must comply with Huckel's law.
- According to this rule, the ring system must have electrons $(4 n+2) \pi$ electrones, where n is a whole number (0, 1, 2, 3, etc.). At this time the ring systems have 2 (n = 0), 6 (n = 1), 10 (n = 2), 14 (n = 3) etc pi electrons.
- Typical examples of aromatic chemicals are benzene, naphthalene, and anthracene.
Related Topics
Characteristics of Aromatic Compounds
1. It has to be Cyclic.
2. You must have (4n + 2) pi Electrons (n = 1,2,3,4, ...)
3. Oppose Add but Select Substitutions.
4. You Must Find the Power of Resonance
Non-benzenoid Aromatic Compounds
- Azulene, which consists of a structure of seven-limbed and five-limbed rings, is a typical non-benzenoid fragrant compound.
- Three effective methods of production have been identified so far as follows: The first method, developed by Ziegler and Hafner, is a ring-salt or pyrylium salt reaction with cyclopentadienides.
- The second, developed by Nzoe and Seto et al. regenerative reactions of tropone containing halogen, methoxy, or tosyloxy groups in area 2 and active methylene compounds, such as cyanoacetates and malonates, where the base is located.
- The latter method, developed by Yasunami and Takase et al., Reactions of oxaazulanones with enzymes.
- The elements of tropolone are also classified as unhealthy benzenoid chemicals. Hinokitiol, the generic site of tropolone, is known to show an anti-bacterial effect.
- Colchicine, an alkaloid compound with a tropone ring, shows strong antitumor effects.
- Tropolone compounds have potential in the field of medicine, especially in anti-cancer drugs.
Also Read -
Some Solved Examples
Question 1: Among the following, the aromatic compounds are :
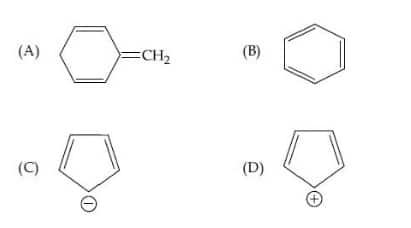
Choose the correct answer from the following options:
1) (A), (B), and (C) only
2) (B), (C), and (D) only
3) (A) and (B) only
4) (correct) (B) and (C) only
Solution:
(A) Non-Aromatic
(B) Aromatic - $6 \pi$ electron
(C) Aromatic - $6 \pi$ electron
(D) Anti-Aromatic - $4 \pi$ electron
The aromatic compounds are (B) and (C) only.
Hence, the answer is option (4).
Question 2: Among the given organic compounds, the total number of aromatic compounds is _______ .
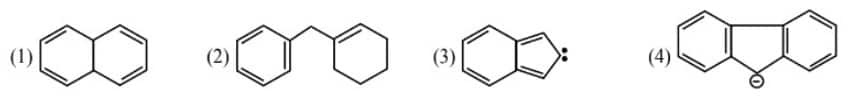
Solution:
Aromaticity -
Aromaticity is defined as "An aromatic compound having a cyclic planar structure with $(4 n+2) \pi$ electrons and has high resonance energy and stability due to delocalization of $\pi$ electrons." Any compound is aromatic if the following conditions are fulfilled:
- It has complete delocalization of $\pi$ electrons.
- Has a high resonance energy.
- Has a conjugate system.
- Has several $\pi$ electrons according to $4 n+2$ or Huckel's rule is $2,6,10,14,18$. Here, $n=0,1,2 \ldots$
- If the number of $\pi$ electrons is 4 " $n$ ' i.e., $4,8,12,16$, it will be anti-aromatic.
- If any of these conditions is not obeyed, it will be non-aromatic.
2, 3, and 4 are aromatic.
Hence, the answer is (3).
Question 3: What is the general formula of aromatic compounds?
1) (correct) $\mathrm{C}_{\mathrm{n}} \mathrm{H}_{\mathrm{n}}$
2) $\mathrm{C}_{\mathrm{n}} \mathrm{H}_{2 \mathrm{n}}$
3) $\mathrm{C}_{2 \mathrm{n}} \mathrm{H}_{\mathrm{n}}$
4) $\mathrm{C}_n \mathrm{H}_{2 n+2}$
Solution :
Aromatic compounds: These compounds consist of at least one benzene ring, i.e, a six-membered carbocyclic ring having alternate single and double bonds. Generally, these compounds have some fragrant odour and are hence named aromatic.
The general formula of aromatic is $C_n H_n$.
Benzene formula is $\mathrm{C}_6 \mathrm{H}_6$ which is $\mathrm{C}_n \mathrm{H}_n$ for $\mathrm{n}=6$.
Hence, the answer is option (1).
Question 4: Which compound (s) out of the following is/are not aromatic?
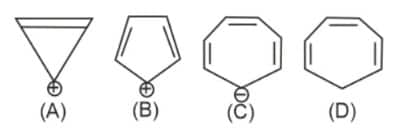
1) (A) and (C)
2) (C) and (D)
3) (correct) (B), (C), and (D)
4) (B)
Solution:
As we have learned,
Aromatic compounds are cyclic, planar, and have $(4 n+2) \pi$ electrons in complete conjugation. If any of these conditions are not satisfied then the compound is not aromatic.
Compound (A) is Aromatic.
Compound $(\mathrm{B})$ is anti-aromatic as it has $4 \pi$ electrons
Compound $(\mathrm{C})$ is non-planar as the negative charge occupies an $s p^3$ hybridized orbital and hence it is non-aromatic
Compound (D) is non-aromatic as it does not have complete conjugation.
Therefore, compounds $\mathrm{B}, \mathrm{C}$, and D are not aromatic.
Hence, the answer is option (3).
Practice More MCQS with the Link iven Below:
Frequently Asked Questions (FAQs)
Fragrant hydrocarbons are compounds that contain benzene as part of their structure, also known as aromatic chemicals. Benzene, with the formula C6H6, is a circular hydrocarbon.
Carbon compounds are directly related to the chemical composition of aliphatic compounds. In aromatic chemicals, Carbon compounds are associated with pi electrons that are bonded in the form of a ring structure.
Common examples of aromatic compounds include:
- Benzene (C6H6)
- Toluene (C7H8)
- Naphthalene (C10H8)
- Aniline (C6H5NH2)
- Phenol (C6H5OH)
Aromatic compounds can be synthesized through several methods, including:
- Electrophilic Aromatic Substitution (EAS): A common reaction where an electrophile replaces a hydrogen atom in the aromatic ring.
- Friedel-Crafts Reaction: A type of EAS involving alkylation or acylation of an aromatic ring with alkyl halides or acyl halides.
- Cyclization reactions: Such as the preparation of aromatic rings from non-aromatic precursors.
Aromaticity is a substance in cyclic organic chemistry, a planar (flat) with resonance bond rings that provide greater stability compared to other geometric or contact arrangements with the same set of atoms.
A heterocyclic compound is a living compound where a non-carbon atom incorporates one of the carbon atoms into the molecule. Nitrogen, oxygen, and sulfur are common hetero atoms.
