Resonance Structures - Lewis Dot, Examples, Rules, Structure, FAQs
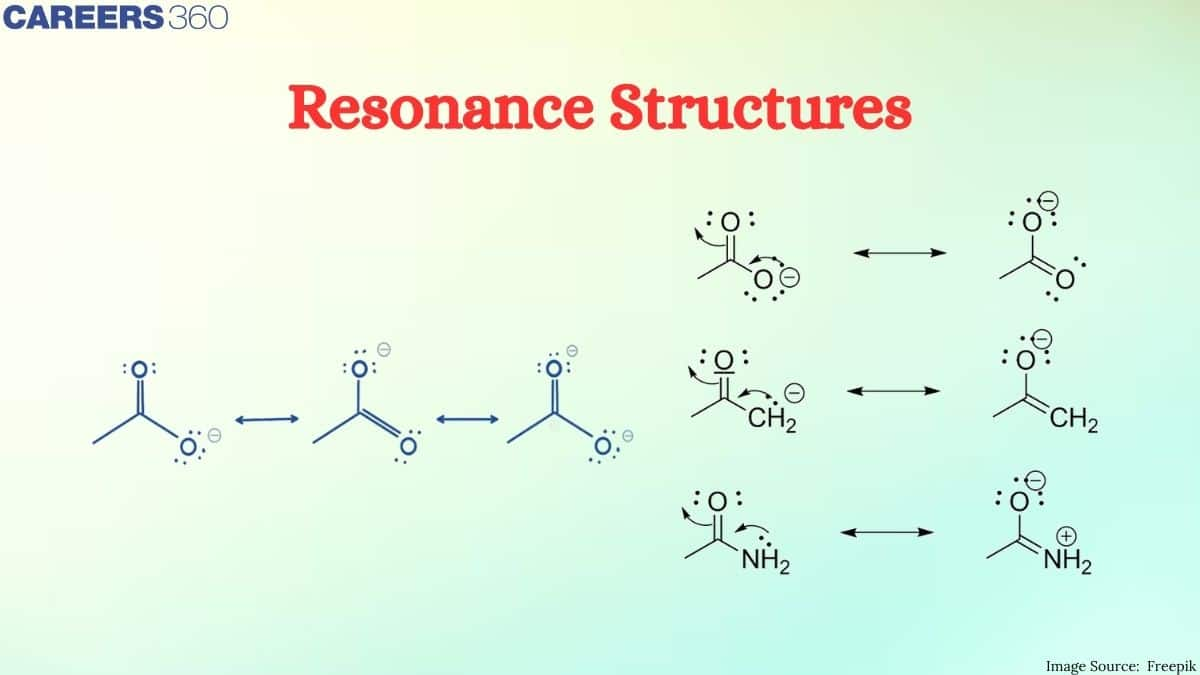
Resonance structures, may be defined as a representation of molecules by more than one valid Lewis structure. Resonance structures come into being when a single Lewis structure is inadequate to fully capture the electron distribution of the molecule, mostly due to electron delocalization across different atoms. Consequently, this goes on to give a resonance hybrid that better explains the actual electronic structure of the molecule.
This Story also Contains
- Resonance Structures
- Types of Resonance Structures
- Practical Applications of Resonance Structures
- Some Solved Examples
- Practice More Questions From the Link Given Below:
- Summary
In this article, we will cover the concept of the Bond Parameters - Resonance structures. This concept falls under the broader category of Chemical Bonding, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
Also Read -
Resonance Structures
Resonance structures are a specific set of two or more Lewis structures all of which describe the electronic bonding in each atom of a molecule or polyatomic ion. In case of certain molecules, a single Lewis structuremcannot explain all the properties of the molecule. The molecule is then supposed to have many structures, each of which can explain most of theproperties of the molecule but none can explain all the properties of the molecule. The actual structure is in betwee of all these contributing structures and is in between of all these contributing structures and is called resonance hybrid and the different individual structures are called resonating structures or canonical forms. This phenomenon is called resonance.
For example, the electronic structure of ozone (O3) can be written by two Lewis structures I and II in which each oxygen atom has an octet of electrons.
.jpg)
If we consider any of these structures I or II, the $
0=0
$ bond (121 pm) should be shorter than $
0-0
$ (148 pm) bond. Thus, the molecule should exhibit two bond lengths. But experiments show that both the oxygen bonds are equal and the bond length (128 pm) is intermediate of single and double bonds. Obviously, this cannot be represented by either of the two Lewis structures shown above.
In order to explain the structure of such types of molecules the concept of resonance was introduced. According to the concept of resonance, whenever a single lewis structure cannot describe a molecule accurately, a number of structure with similar energy, positions of nuclei, bonding and non-bonding pairs of electrons are taken as the canonical structure of the hybrid which describes the molecule accurately. So, the actual structure of ozone is intermediate of structure I and II and is called resonance hybrid. The structure I and II are called resonating structures, contributing structures or canonical forms. The resonance between canonical forms can be represented by a double headed arrow ($
\leftrightarrow
$). Although it is not possible to draw the Lewis structures for the resonance hybrid, but structure III represents the structure of O3 more accurately.
It may be borne in mind that actual structure does not oscillate between the resonating forms and has its individual identity.
Related Topics link:
- Classification Organic Compounds
- Diastereomers
- Structure of Benzene
- Functional Groups
- Sigma and Pi Bond
Bond Order
Bond order is defined as the number of bonds between the two atoms in a molecule. For example, in an H-H or H2 molecule, there is only a single bond present thus its bond order is 1. Further, in O=O or O2, the bond order is 2 as it has 2 bonds between the oxygen atoms.
Resonance
The nitrite anion can have two possible structures with the atoms in the same positions. The electrons involved in the N–O double bond, however, are in different positions as shown below.

If nitrite ions do indeed contain a single and a double bond, we would expect the two bond lengths to be different. A double bond between two atoms is shorter than a single bond between the same two atoms. Experiments show, however, that both N–O bonds in NO2− have the same strength and length, and are identical in all other properties.
It is not possible to write a single Lewis structure for NO2− in which nitrogen has an octet and both bonds are equivalent. Instead, we use the concept of resonance: if two or more Lewis structures with the same arrangement of atoms can be written for a molecule or ion, the actual distribution of electrons is an average of that shown by the various Lewis structures. The actual distribution of electrons in each of the nitrogen-oxygen bonds in NO2− is the average of a double bond and a single bond. We call the individual Lewis structures resonance forms. The actual electronic structure of the molecule is called a resonance hybrid of the individual resonance forms. A double-headed arrow between Lewis structures indicates that they are resonance forms. Thus, the electronic structure of the NO2− ion is shown as:

Resonance Hybrid
It is the average of the resonance forms shown by the individual Lewis structures or canonical structures.
Types of Resonance Structures
Resonance structures may be characterized by some main points which have to be addressed.
1. Equivalent and Non-equivalent Resonance Structures: Resonance structures that contribute to the same amount in the resonance hybrid are called equivalent resonance structures. Those that don't contribute equally are non-equivalent structures. The nitrate ions all have equivalent resonance structures, so the average of the nitrogen-oxygen bond order is 1.33. In ozone, the two resonance structures are not equivalent, so one will predominate, or be more prevalent, in the resonance hybrid.
2. Stability of Resonance Structures Not all resonance structures are equal. Some are more stable than others due to formal charge, electronegativity, and the octet rule. Structures that result in the least formal charges possible while still maintaining full octets for all atoms are generally more stable and, therefore, contribute more significantly to the resonance hybrid.
3. Fractional Bond Orders: It is extremely common for the resonance structure to be fractional, thus indicating that the bonds are neither purely single nor double bonds. In the case of benzene, electron delocalization between carbon atoms gives a bond order of 1.5.
These characteristics of resonance structures enable an understanding of how electron delocalization could cause changes in molecular geometry and reactivity.
Also Read:
Practical Applications of Resonance Structures
The concept of resonance structures stretches far into related fields of chemistry and beyond.
1. Organic Chemistry: Resonance forms the basis for the stability and reactivity of an organic molecule. Using benzene, resonance stabilization will make it less reactive than an alkene because delocalized electrons increase its stability. This resonance is quite critical in the prediction of products that are expected to occur in a reaction.
2. Pharmaceuticals: An example, in the case of drug design, is how resonance structures can modulate the activity of compounds at hand. Resonance stabilization is responsible, for instance, for the improved binding capacity of some functional groups present in drugs to their respective targets. Indeed, the role of resonance goes as far as medicinal chemistry itself.
3. Materials Science: Resonance structures can be used to explain such diverse properties of a material, for example, polymers and nanomaterials. In most conjugated systems, electron delocalization provides special optical or electronic properties used in designing new materials.
4. Biochemistry: Many biological molecules, such as enzymes and nucleic acids, are in resonance. Knowledge such as this about resonance structures of these molecules gives insight into the type of function they might provide or interaction type within a biological system.
Tying it together, resonance structures are useful theoretical devices; they also have applications across the disciplines in science. Resonance allows a scientist to make more accurate predictions concerning the behavior of molecules, design more potent drugs, and invent new materials in general.
Recommended Topic Video on(Resonance Structure)
Some Solved Examples
Example 1:
Which of the following resonating structures is not correct for CO2?
1)
2)
3)
4)
Solution
The Oxygen atom forming a triple bond cannot have a negative charge on it as Oxygen will then have 10 electrons in the valence shell which is not possible
Hence, the answer is Option (3)
Example 2
Question: The bond order of CO and O₂ is:
1) 3 and 2
2) 3 and 2.5
3) 3 and 1.3
4) 3 and 3.5
Solution: Bond order is the number of chemical bonds between a pair of atoms in a molecule. For CO, the bond order is 3, indicating a triple bond between carbon and oxygen. For O₂, the bond order is 2, indicating a double bond between oxygen atoms.
Hence, Option (1) is correct.
Example 3:
Which of the following conditions is not correct for resonating structures?
1) The contributing structures must have the same number of unpaired electrons.
2) The contributing structures should be so written that unlike charges reside on atoms that are far apart.
3) The contributing structures should have similar energies.
4) The positive charge should be present on the electropositive element and the negative charge on the electronegative element.
Solution: Resonance structures with similar charges on adjacent atoms are insignificant due to electrostatic repulsion and are thus unstable. There is no rule that positive and negative charges on atoms should be far apart.
Hence, the correct option is (2).
Example 4:
The bond order and the magnetic characteristics of CN- are:
1) 3, paramagnetic
2) $\begin{array}{r}1 \\ 2- \\ 2\end{array}$, diamagnetic
3) 3, diamagnetic
4) $\begin{array}{r}1 \\ 2- \\ 2\end{array}$, paramagnetic
Solution: The bond order of CN⁻ is 3, as there is a triple bond between carbon and nitrogen. All the electrons are paired in this ion, making it diamagnetic.
Hence, the answer is Option (3).
Example 5:
Point out an incorrect statement about resonance
1) Resonance structures should have equal energy
2) In resonance structures, the constituent atoms should be in the same position
3) In resonance structures, there should not be the same number of electron pairs
4) Resonance structures should differ only in the location of electrons around the constituent atoms
Solution
We know these facts-
- Resonance structures should have equal energy.
- In resonance structures, the constituent atoms should be in the same position.
- In resonance structures, there should be the same number of electron pairs.
- Resonance structures should differ only in the location of electrons around the constituent atoms
Thus the incorrect statement about the resonance structure is C.
Hence, the answer is the option (3).
Example 6:
Resonance in carbonate ion CO32- is
 Which of the following is true?
Which of the following is true?
1) All these structures are in dynamic equilibrium with each other.
2) It is possible to identify each structure individually by some physical or chemical method.
3) Each structure exists for equal amount of time.
4) CO32- has a single structure i.e., resonance hybrid of the above three structures.
Solutions:
Resonating structure are hypothetical and resonance hybrid is a real structure which is weighted average of all the resonating struture.
Hence, the answer is the option (4).
Practice More Questions From the Link Given Below:
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT notes Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and techniques
Summary
Resonance structures are an important feature in chemistry to try and indicate the delocalization of electrons within a molecule or even polyatomic ions. The concept of resonance shows that there will be more than one valid Lewis structure that details the bond and stability of these compounds—something a single structure cannot fully explain. Some other types of resonance structures, like equivalent and nonequivalent types, carry immense information regarding the behavioral history of a molecule.
Also Check
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Yes. Resonance structures may have different distributions of formal charges, but the total charge of each must be the same. You can move electrons to shift formal charges among atoms, as long as the overall charge is preserved.
No. Some resonance forms are minor contributors (less stable) and contribute less to the hybrid. Major contributors are those with favorable formal charge distribution and full octets.
Resonance structures should not violate the octet rule for second‑period elements (C, N, O, F). In some cases with third‑period or higher atoms, expanded octets may appear, but those must be treated carefully.
Radical species (with unpaired electrons) can also have resonance, where the unpaired electron delocalizes. For ions, resonance helps distribute charge over several atoms, often stabilizing the ion.
Resonance is possible when there is a conjugated system: alternating single and multiple bonds, or a lone pair adjacent to a π bond, or when charge delocalization is feasible. Also, the movement of electrons (not atoms) should not violate octet rules (for 2nd row elements).
The resonance form that is more stable (lowest in energy) contributes more. Factors include: minimal formal charges, negative charges on more electronegative atoms, full octets, and structure symmetry.
Moving atoms (instead of electrons)
Violating total electron count or charge
Incorrect use of arrow pushing (curved arrows must show movement of electron pairs, not atoms)
Forgetting to include lone pairs or formal charges
Drawing resonance forms that are too unstable (like placing negative charge on a less electronegative atom)
Different Lewis structures for a single chemical compound describing the delocalisation of electrons is called a resonance structure.
The overall description of resonance structures is called a resonance hybrid.
Electrons are not associated with a single atom and hence they spread over to attain stability.