Bcc Fcc Primitive Cubic Unit Cell - Definition, Structure, Types, FAQs
Have you ever wondered how atoms are actually arranged inside a solid? Why do some metals like iron have one type of packing while others like copper or sodium have a different one? You will find all these answers by reading this article on FCC, BCC, and the primitive unit cell. The arrangement of atoms inside the material influences its properties. Like gold is very soft, malleable, and ductile, iron is tough and strong, while aluminium is light in weight. The difference in the properties is only because of the arrangement of atoms inside their unit cells.
This Story also Contains
- Unit Cell
- Types of Unit Cell
- Primitive Cubic Unit Cell
- Body-centered Unit Cell (BCC)
- Face-centered unit Cell (FCC)
- What is Lattice?
- Some Solved Examples

The unit cell is the smallest repetitive unit of the crystal lattice or a crystal structure. The 3D arrangement of atoms, molecules, or ions inside the given crystal is called a crystal lattice. There are three main types of unit cells: Simple cubic cell, Body-centered cubic unit cell, and Face-centered cubic unit cell. The unit structure of iron is BCC, which is why it’s strong, while the unit structure of Gold and aluminium is FCC, making them soft and malleable.
Unit Cell
The smallest group of atoms has the same number of crystals, and where the entire lattice can be formed by three dimensions is called the Cell Unit. Crystalline Solids exhibit a normal and repetitive pattern of existing particles.
Representation of the three-dimensional design of the particles present in the crystal, in which each particle is presented as a point in space known as a crystal lattice.
FCC structure
FCC (face-centered cubic): Atoms are usually arranged at the corners and even at the center of the surface of each given cell. Atoms are considered to affect the diagonals of the face. 4 atoms in a single unit cell. Atoms are arranged in the corners of the cube, and another atom is in the center of the cube.
BCC structure
The BCC unit cell has a total number of two atoms, one in the center and one at each corner. In the FCC system, there are also eight atoms in the corners of a cell with one atom centered on each surface. The atom on the surface is shared with a nearby cell.
What are an FCC structure and a BCC structure?
The most direct difference between FCC crystals and BCC is in the atomic systems. The cubic structure in the center of the face has an atom in all 8 positions, and in the center of all 6 faces. The body-centered cubic structure has atoms in all eight corner positions, and one is in the center of the cube.
Types of Unit Cell
Multiple unit cells together form a crystal lattice. Physical particles such as atoms and molecules also exist. Each lattice point remains with such particles.
1. First Cubic Cell
2. Body-centered Body Unit Cell
3. A cell unit in the center of the face
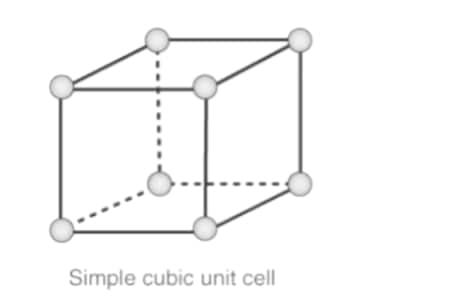
Primitive Cubic Unit Cell
In the first cell of the cubic unit, atoms are found only in the corners. Every atom in a corner is shared between cells in eight adjacent units. There are four unit cells in the same layer as 4 in the upper (or lower) layer. Thus, a single unit of a cell has only 1 / 8th of an atom. Each subdivision in each of the following figures represents the particle center in that particular position and not its size. This building is known as the open-air building.
Atoms in the first phase of the simple cubic unit cell are found only in the corners
All the atoms in the corners are divided between the cells of the eight adjacent units
Four unit cells exist in the same layer
One unit cell is in the upper / lower layer
Thus, a single cell unit has only 18 atoms
Each subdivision in each of the following figures represents the particle center in that particular position and not its size
In each cell of the cubic unit, there are 8 atoms in the corners. Therefore, the total number of atoms in a single cell is
8 × 1/8 = 1 atom.
|
Related Topics |
Body-centered Unit Cell (BCC)
The BCC unit cell has atoms in each corner of the cube and the atom in the center of the structure. According to this structure, the atom in the center of the body entirely belongs to the cell unit in which it is located. In the BCC unit cell, every corner has atoms. There is one atom in the center of the building. At the bottom of the drawing is an open structure.
According to this, the atom of the structure in the physical organs is entirely the cell of the unit in which it is located.
Number of atoms in a BCC cell:
Therefore, in the BCC cell, we have:
8 x 1/8 corners with each atom = 8 × 1/8 = 1 atom
1 physical center atom = 1 × 1 = 1 atom
Hence, here the total number of atoms that are present per cell unit = 2 atoms.
Face-centered unit Cell (FCC)
The FCC unit cell contains atoms in all corners of the crystal lattice and in the center of each cube surface. An atomic surface atom is divided between cells in two adjacent units, and only 1/2 of each atom is in each cell.
In the FCC unit cell, atoms are present in all corners of the crystal lattice
Also, there is an atom at the center of the whole surface of the cake
This atomic center is divided between cells in two adjacent units
Only about 12 atoms are part of a cell
Number of atoms in an FCC cell
a) 8 x 1/8 corners with each atom = 8 × 1/8 = 1 atom
b) Six surface atoms × 1/2 atom per unit of cell = 3 atoms
Therefore, the total number of atoms in a cell = 4 atoms
Therefore, in the cellular unit centered on the surface, we have:
8 x 1/8 corners with each atom = 8 × 1/8 = 1 atom
Six atoms centered on the surface × 1/2 atom per unit of cell = 3 atoms
Therefore, the total number of atoms in a cell = 4.
What is Lattice?
A lattice is a three-dimensional structure, a series of periodic points, on which a crystal is formed. In 1850, M. A. Bravais showed that similar points can be arranged geographically to produce 14 types of standard patterns. These 14 space fragments are known as Bravais lattices.
The crystal lattice of solidity can be defined according to its unit cell. A crystal lattice is made up of a very large number of unit cells, where every lattice point resides in a single particle. A unit cell can be seen as a three-dimensional structure consisting of one or more atoms.
We can see the volume of this cell unit in terms of cell unit size. For example, if we have a single edge cell “a”, the unit cell volume can be given as “a3”. The unit size of a cell is given as a measure of the size and volume of the cell. The unit size of a cell is equal to the product of the number of atoms in the cell and the size of each atom in the unit cell.
Quantity of cell unit = number of atoms per cell unit × size of each atom = z × m
Where, z = number of atoms in a cell,
m = Mass for each atomic mass
Atomic mass can be given with the help of Avogadro's number and molar mass as:
MNA
Where, M = molar mass
NA number = Avogadro
Cell unit volume, V = a3
=> Multiple cell unit = maximum cell unit unit cell
=> Cell unit quantity $=m V=z \times m a^3=z \times M^3 \times N_A$
Therefore, with the knowledge of the number of atoms in the unit cell, the marginal length and the molar size can determine the cell density of the unit.
A general description of the cell unit size of the various cases is found below:
1. The first cell unit: In the cell of the first unit, the number of atoms in a unit is equal to one. Therefore, the size is given by:
Maximum cell unit = 1 × Ma3 × NA
2. Cells centered in a cubic unit: In a cell-centered cubic unit cell, the number of atoms in a cell is equal to two. Therefore, the size is given by:
Maximum cell unit = 2 × Ma3 × NA
3. Cube-centered cubic unit cell: In a cell-centered cubic unit cell, the number of atoms in a unit cell is equal to four. Therefore, the size of the cell unit is given as:
Maximum cell unit = 4 × Ma3 × NA
Also check-
Some Solved Examples
Question 1: Which of the following statements is true regarding primitive unit cells?
1) They contain one or more constituent particles at positions other than corners.
2) (correct) They contain constituent particles only at the corner positions.
3) They contain one constituent particle at the body-centre besides the ones at the corners.
4) They contain one constituent particle present at the centre of each face besides the ones at the corners.
Solution:
Primitive and Centred Unit Cells
Primitive Unit Cells
When constituent particles are present only on the corner positions of a unit cell, it is called a primitive unit cell.
Centred Unit Cells
When a unit cell contains one or more constituent particles present at positions other than corners in addition to those at corners, it is called a centred unit cell. Centred unit cells are of three types:
- Body-Centred Unit Cells: Such a unit cell contains one constituent particle (atom, molecule or ion) at its body-centre besides the ones that are at its corners.
- Face-Centred Unit Cells: Such a unit cell contains one constituent particle present at the centre of each face, besides the ones that are at its corners.
- End-Centred Unit Cells: In such a unit cell, one constituent particle is present at the centre of any two opposite faces besides the ones present at its corners.
Primitive unit cells are defined as unit cells that contain only constituent particles at the corner positions. This means that there are no particles at any other positions within the unit cell. Therefore, option b) is the correct statement. Option a) describes centred unit cells, while options c) and d) describe specific types of centred unit cells (body-centred and face-centred, respectively).
Hence, the answer is option (2).
Question 2: A metal crystallises with an FCC lattice. The edge length of the unit cell is 408 pm. The diameter of a metal atom is
1) (correct) 288 pm
2) 408 pm
3) 144 pm
4) 204 pm
Solution:
For the FCC lattice,
$
\begin{aligned}
& 4 r=a \sqrt{2} \\
& r=\frac{a \sqrt{2}}{4}=\frac{408}{2 \sqrt{2}}=144 \mathrm{pm} \\
& \text { diameter }=2 r=144 \times 2=288 \mathrm{pm}
\end{aligned}
$
Hence, the answer is option (1).
Question 3: In a simple cubic cell, each point on a corner is shared by
1) 2 unit cells
2) 1 unit cell
3) (correct) 8 unit cells
4) 4 unit cells
Solution:
Crystal Lattices and Unit Cells
A portion of the three-dimensional crystal lattice and its unit cell.
In the three-dimensional crystal structure, a unit cell is characterised by:
(i) its dimensions along the three edges a, b, and c. These edges may or may not be mutually perpendicular.
(ii) angles between the edges, α (between b and c), β (between a and c), and γ (between a and b). Thus, a unit cell is characterised by six parameters a, b, c, α, β, and γ.
Primitive Unit Cells
When constituent particles are present only on the corner positions of a unit cell, it is called a primitive unit cell.
Solution
As we learnt in
Primitive unit cell -
In a primitive unit cell, constituent particles are present only in the corner positions of the unit cell.
In the simple cubic cell, each point on a corner is shared by 8 unit cells.
Hence, the answer is option (3).
Question 4: The smallest repeating pattern that, when repeated in 3-D, results in the crystal of the substance is called:
1) Space lattice
2) Crystal lattice
3) (correct) Unit cell
4) Bravais lattice
Solution:
The unit cell is the smallest repeating unit in the crystal lattice, which has all the properties of the crystal.
Hence, the answer is option (3).
Question 5: A compound formed by the elements X and Y has a cubic structure in which X atoms are at the corners of the cube and Y atoms are at the face centers. Calculate Zeff.
1) (correct) 4
2) 3
3) 5
4) 2
Solution:
Number of effective $X$ atoms $=8 \times \frac{1}{8}$ per corner atom share
Thus, effective $X$ atoms $=1$ atom/unit cell
Now, the number of effective Y atom corners $\times \frac{1}{2}$ per face atom share
Thus, effective Y atoms - 3 atoms/unit cell
Hence, $Z_{e f f(8 X+Y)}=1+3=4$
Hence, the correct answer is option (4).
Practice More Questions With The Link Given Below
| Crystal Lattices and Unit Cells practice question and MCQs |
| Close Packed Structures practice question and MCQs |
Frequently Asked Questions (FAQs)
The packing efficiency of BCC is about 68%, while FCC boasts a higher packing efficiency of around 74%. Packing efficiency calculates the volume occupied by the atoms relative to the total volume of the unit cell.
A BCC unit cell effectively contains 2 atoms. There are 8 atoms located at the corners (contributing 1/8 each) and 1 atom in the center, totaling 2 atoms when added together.
A primitive unit cell is the smallest repeating unit of a crystal lattice that can be used to construct the entire lattice through translation. It contains one or more atoms and reflects the symmetry and structure of the crystal.
BCC and FCC are two types of crystal structures found in metals and other materials. BCC has atoms positioned at the corners of a cube with one atom at the center, while FCC has atoms at each corner and the centers of each face of the cube.
An FCC unit cell contains 4 atoms. It has 8 corners (contributing 1/8 each) and 6 face-centered atoms (contributing 1/2 each), which totals to 4 atoms.
