Alloy - Definition, Examples, Types of Alloys with FAQs,
Have you ever thought about why pure metals are rarely used in making bridges, airplanes, or even the coins in your pocket? Why is it that adding just a small amount of another element can turn a soft, malleable metal into a strong, durable material? The answer is alloys this transformation happen by the formation of alloys. Alloys are materials made by combining two or more metals—or sometimes a metal and a non‑metal—while melted. The result usually has different traits than the individual ingredients.
This Story also Contains
- Examples Of Alloys
- Types Of Alloys
- Interstitial Alloys
- Bell Metal
- Bronze
- Nichrome
- Steel
- Some Solved Examples

Compare to pure metals, alloys often become stronger and harder. For example, red gold is made by mixing copper with gold, giving it a warm reddish color. White gold is formed by blending silver—or metals like nickel or palladium—with gold, creating a silvery-white alloy. These mixed metals are very useful in jewelry and everyday tools because they combine improved strength with pleasant colors and finishes.
Types Of Alloys
They are mainly classified into the following types:
-
Ferrous Alloys – Contain iron as the main element.
-
Examples: Steel, Cast Iron.
-
-
Non-Ferrous Alloys – Do not contain iron.
-
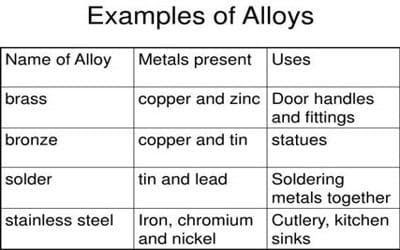
Examples: Brass (Copper + Zinc), Bronze (Copper + Tin), Duralumin (Aluminum + Copper).
-
-
Substitutional Alloys – Atoms of the solute replace host metal atoms in the crystal lattice.
-
Example: Brass (Copper + Zinc).
-
-
Interstitial Alloys – Smaller atoms fit into the spaces (interstices) of the host metal lattice.
-
Example: Steel (Iron + Carbon).
-
-
Miscellaneous / Special Alloys – Designed for specific properties like corrosion resistance, low melting point, or superconductivity.
-
Examples: Solder (Tin + Lead), Nichrome (Nickel + Chromium).
-
They’re also classified by phase count: homogeneous alloys have a single uniform phase, while heterogeneous alloys consist of two or more distinct phases within the material.
Examples Of Alloys
Babbitt metal, also known as bearing metal, is a soft alloy first created in 1839 by American inventor Isaac Babbitt.
It typically contains around 90 % tin, 7 % antimony, and 3 % copper. This combination yields a metal matrix with tiny hard crystals in a softer base, letting it support heavy loads while embedding debris and preventing shafts from seizing if lubrication fails.
Its smooth, low‑friction surface makes it ideal for lining simple bearings, especially when matched with steel, reducing wear and improving performance
The chemistry of alloys
A strong electron microscope can reveal the atoms inside a metal. Then the atoms are grouped in a regular arrangement called a crystalline lattice.
Related Topics,
Types Of Alloys
Here we discuss substitutional alloy and instestial alloys
Substitution Alloy
A substitutional alloy is made when atoms of one metal swap places with atoms of another in the metal’s crystal structure. This only works when the atoms are about the same size and chemically similar—often because they're close together on the periodic table.
A common example is brass, which is mostly copper with about 10–35% of the copper atoms replaced by zinc. Copper and zinc are a good match because their atoms are similar in size and structure, so zinc fits neatly into the copper lattice without disrupting it.
Substitutional alloys like brass keep the original metal’s crystal pattern but gain improved traits—such as more strength, better resistance to wear, and sometimes enhanced electrical or thermal characteristics.
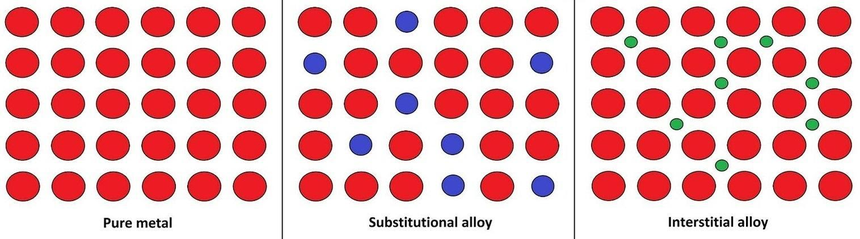
Interstitial Alloys
Alloys with interstitial atoms
Certain alloys form when the added element has much smaller atoms than the main metal. These tiny atoms slip into the empty spaces, or "interstices," within the metal’s crystal structure, creating an interstitial alloy. A common example is steel, where a few carbon atoms fit between the iron atoms in its crystal lattice.
Bell Metal
Bell metal is a special kind of bronze used mainly to cast bells and cymbals. It contains about 78–80% copper and 20–22% tin, giving it a stiff and resonant quality that produces clear, long-lasting sounds. Aside from bells, it's also used for decorative items, cookware, and even mechanical parts like bearings and valve components . Its higher tin content strengthens the alloy, makes it more elastic, and helps it resist wear and corrosion. Over time, a natural greenish patina forms on its surface, which protects it from further oxidation.

Bronze
Bronze is primarily a mix of copper and tin—usually about 88% copper and 12% tin—sometimes with tiny amounts of metals like manganese, aluminum, nickel, silicon, phosphorus, or arsenic to improve its properties.
It's much harder and stronger than pure copper and also more resistant to corrosion. These qualities make it easier to machine and shape. As a result, bronze is commonly used to make items like medals, coins, trophies, heavy gears, tools, and electrical parts . Its durability and toughness suit various industrial and decorative applications.

Nichrome
Nichrome is an alloy made mainly of nickel and chromium, usually around 80% nickel and 20% chromium, with small amounts of iron or other metals. It’s best known as resistance wire, where it converts electricity into heat, making it ideal for devices like toasters, space heaters, hair dryers, and industrial furnaces.
Nichrome is also used in dental fillings, igniters for fireworks and model rockets, and in foam-cutting tools. Thanks to its high melting point (around 1,400 °C), resistance to oxidation, and stable electrical resistance, it withstands repeated heating and cooling cycles without degrading.

Steel
Steel is mainly an alloy of iron and carbon, but many types also include other metals like chromium, manganese, nickel, sulfur, phosphorus, copper, or molybdenum. Iron makes up most of its weight—at least around 75%. The amount of carbon and added elements varies by steel type.
For instance, stainless steel typically contains about 85–88% iron, at least 10.5% chromium, and less than 1.2% carbon. These added elements give steel extra strength, resistance to rust, and toughness, making it more durable and versatile than pure iron.

Some Solved Examples
Question: 1 Which of the following is an example of an alloy?
A) Copper
B) Brass
C) Aluminium
D) Zinc
Solution:
Brass is an alloy of copper and zinc, whereas copper, aluminium, and zinc are pure metals.
Hence, the correct answer is option (b)
Question: 2 Stainless steel is an alloy of iron with:
A) Carbon and Nickel
B) Chromium and Nickel
C) Zinc and Tin
D) Copper and Tin
Solution:
Stainless steel contains iron, chromium, and nickel, which provide corrosion resistance and strength.
Hence, the correct answer is option (b)
Question: 3 Solder, used for joining electrical wires, is an alloy of:
A) Copper and Zinc
B) Lead and Tin
C) Aluminium and Copper
D) Iron and Carbon
Solution:
Solder is an alloy of lead and tin with a low melting point, making it ideal for joining metals.
Hence, the correct answer is option (b)
Practice More Question With Link Given Below
| Allotropic Form of Carbon(Diamond) practice question and mcqs |
| Allotropic Form of Carbon(Graphite) practice question and mcqs |
| Allotropic Form of Carbon(Fullerenes) practice question and mcqs |
Also read -
Frequently Asked Questions (FAQs)
The following are some commercially important alloys:
steel, Nichrome, Bronze, Brass, Duralumin, solder
The following is a list of five common alloying elements.
Chromium
Vanadium
Molybdenum
Nickel
Manganese
Alloys can rust, but it depends on their composition.
Rust-resistant alloys:
Stainless steel: Contains at least 10.5% chromium, forming a protective oxide layer that resists rusting.
Copper alloys: Do not rust due to minimal iron content; instead, they develop a protective patina.
Alloys that can rust:
Iron-based alloys: If an alloy contains iron, it can rust when exposed to moisture and oxygen.
In summary, alloys without iron or with added corrosion-resistant elements can prevent rusting. However, those containing iron may rust under certain conditions.
Alloys offer several advantages: they’re typically stronger and harder than pure metals, resist corrosion well (like stainless steel), handle heat better (e.g., in aerospace), and can be tailored for specific needs—making them versatile and efficient over time. The downsides include more expensive and complex production, lower electrical and thermal conductivity, recycling difficulties, and sometimes reduced ductility or weldability.
The common examples of alloys are brass, stainless steel, bronze, aluminum alloys, copper-nickel alloys, titanium etc.
The main advantages of using alloys include:
- Improved Strength: Alloys often possess enhanced tensile strength and hardness compared to their component metals.
- Corrosion Resistance: Many alloys are designed to resist rust and chemical degradation.
- Ductility and Malleability: Alloys often maintain or improve the workability of metals, allowing for easier shaping and manufacturing.
- Cost-Effectiveness: Using cheaper metals or combining metals can help reduce costs while enhancing performance.
Alloys are used in a wide range of applications, including:
- Construction: Steel is commonly used in building materials and structures.
- Aerospace: Aluminum alloys are used for aircraft due to their lightweight and strength.
- Automotive: Various alloys are used for engine components, wheels, and body panels.
- Marine: Bronze and other corrosion-resistant alloys are used in shipbuilding and marine applications.
Common types of alloys include:
- Steel: An alloy of iron and carbon, often with other elements to improve strength and corrosion resistance.
- Brass: An alloy of copper and zinc, known for its malleability and acoustic properties.
- Bronze: An alloy primarily of copper and tin, known for its resistance to corrosion and wear.
- Aluminum Alloys: Mixtures of aluminum with elements like copper, magnesium, or silicon, are used in various applications due to their lightweight and strength.
Alloys are metal alloys or metal alloys with additional elements. Certain other metals/elements can be added to metals in certain ratios to impart certain properties or to strengthen some of their existing properties, resulting in alloys. Pure aluminium, for example, is a rather soft metal. Copper is also a soft metal. When aluminium is alloyed with copper, however, the resulting alloy has a far higher strength than the parent metals.
A blend of two or more elements, at least one of which is a metal, is known as an alloy. Some alloys, such as brass and bronze, are presumably familiar to you. Brass is a copper and zinc alloy. Bronze is a copper and tin alloy.