Schottky Defect - Definition, Examples, Diagram, Formula, Characteristics, FAQs
Have you ever wondered why some crystals may appear less dense or exhibit unusual properties compared to others? We can answer this question by saying that there is a defect in the crystal. We can explain it in terms of defects. One such defect is the Schottky defect, a type of point defect that occurs in ionic crystals. Schottky defect arises when an equal number of cations and anions are missing from the crystal lattice, and how this missing matter affects the density and stability of ionic solids!
This Story also Contains
- What Is Schottky Defect?
- Visual Representation of Schottky Defect
- Schottky Defect Characteristics
- Properties Of The Schottky Defect
- Schottky Defects Formula
- Schottky Defect Examples
- Differentiate Between Frenkel and Schottky Defects
- Some Solved Examples
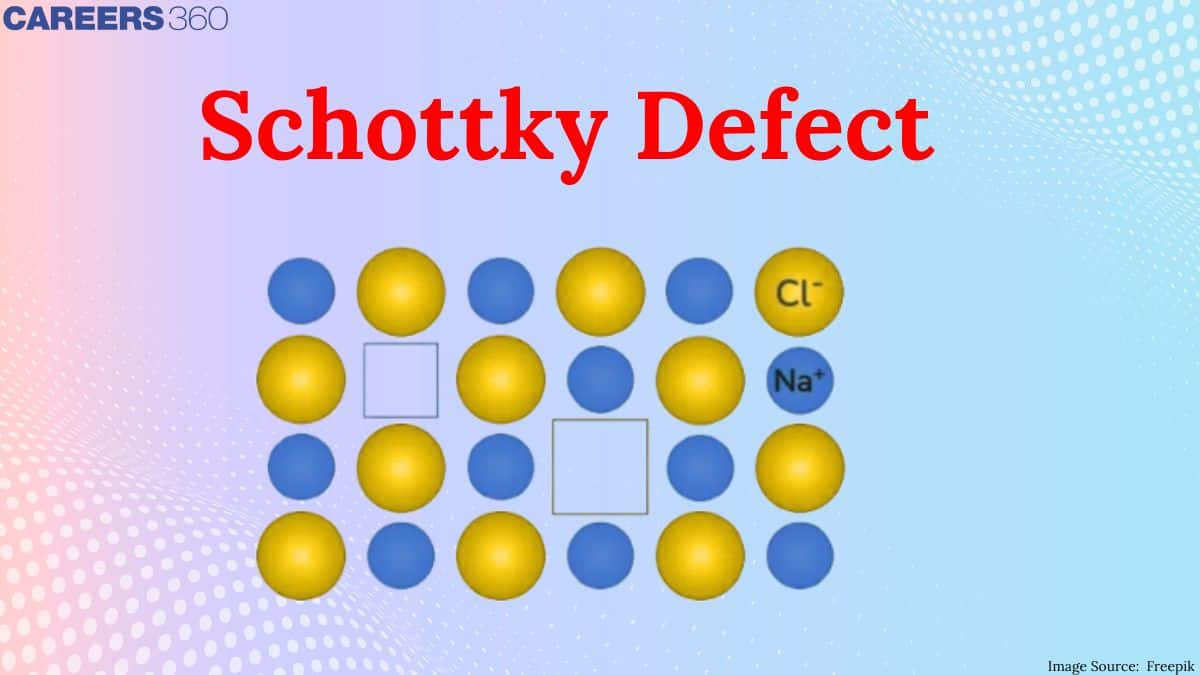
What Is Schottky Defect?
When an equal number of cations and anions are missing from the lattice, a Schottky defect occurs. Lattice structures (also known as crystals) are far from flawless, just like the human body. Our bodies try hard to make things proportionate, but sometimes our right foot is bigger than our left; similarly, crystals try to organise their ions in a tight arrangement, but sometimes an ion slips to another position or just disappears.
Realistically, it is to be expected that crystals will deviate from their normal sequence (not surprising considering defects occur at temperatures greater than 0 K). There are numerous ways for a crystal to lose its order (and hence develop faults); these defects are classified as Point Defects, Line Defects, and other types of defects.
Visual Representation of Schottky Defect
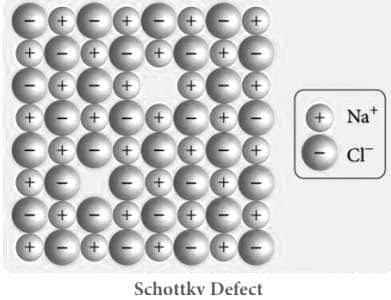
Schottky Defects in Specific Areas
Point defects in lattice structures (or crystals) can be one of two types:
atoms or ions that have left their original location (thus creating vacancies).
Interstitials are formed by atoms or ions slipping into the small gaps between other atoms or ions; as atoms or ions in crystals occupy interstitials, they intrinsically become (make) interstitials.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 1 The Solid State
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 1 The Solid State
Schottky Defect Characteristics
When heat is applied to crystals of ionic substances, Schottky defect forms. Thermal oscillations occur inside the crystal as the temperature rises due to the heat. As a result, gaps appear in the crystal pattern. The availability of ions in chemical compounds causes voids to form.
The "n" ions of X and the "m" ions of Y, for example, will exit the lattice to generate a vacancy in an ionic compound with the formula XnYm. A Schottky cluster is a collection of these job openings.
Properties Of The Schottky Defect
Vacancy Formation:
-
In a Schottky defect, a cation and an anion are both missing from the crystal lattice at specific sites, creating vacancies.
-
The number of cations and anions missing is always equal, maintaining the overall neutrality of the crystal.
Density Decrease:
-
The removal of ions from the lattice results in a reduction in the overall density of the crystal. Since there is a loss of matter without a corresponding increase in the crystal’s size, the crystal becomes less dense compared to a perfect crystal.
Electrical Neutrality:
-
The Schottky defect ensures electrical neutrality because the number of positive and negative charges removed is the same. This distinguishes it from other defects that might cause an imbalance in charge.
Occurs in High Symmetry Crystals:
-
Schottky defects are most commonly found in ionic solids that have a high symmetry and closely packed structures, such as NaCl or KCl.
Low Temperature Formation:
-
Schottky defects typically form at high temperatures, as thermal energy is required to overcome the lattice energy and create vacancies.
Impact on Ionic Conductivity:
-
Schottky defects can increase the ionic conductivity in the material, as the missing ions create vacancies that can facilitate the movement of ions through the lattice.
Formation Energy:
-
The formation of Schottky defects requires a certain amount of energy, known as the vacancy formation energy, which is related to the bond strength between the cations and anions in the lattice.
Non-Stoichiometric:
-
Because of the missing ions, Schottky defects result in a non-stoichiometric crystal structure, meaning the actual number of ions may deviate slightly from the ideal stoichiometric ratio in the bulk material.
Schottky Defects Formula
Heat is used to create Schottky defects in solid crystals. The following formula can be used to compute the faults at a given temperature:
Ns×≈Ne -Hs/2RT
Were,
At temperature T, Ns = number of Schottky defects per unit volume (in Kelvins)
Hs is the enthalpy of producing a single flaw.
R is the gas constant.
T stands for absolute temperature (in K)
The following formula can be used to calculate N:
N= (Ionic Crystal Compound Density NA) / (Molar Mass of Ionic Crystal Compound)
Schottky Defect Examples
A Schottky defect is a type of crystal defect that occurs mostly in strongly ionic or highly coordinated substances. The size difference between the anions and the cations in the compound's lattice is quite modest.
Examples of Schottky defect
- Sodium chloride (NaCl)
- potassium chloride (KCl)
- potassium bromide (KBr)
- caesium chloride (CsCl) and silver bromide are some examples of salts with Schottky faults (AgBr).
The following formula can be used to calculate N:
N= (Ionic Crystal Compound Density NA) / (Molar Mass of Ionic Crystal Compound)
Differentiate Between Frenkel and Schottky Defects
Despite the fact that both the Schottky and Frenkel defects are point defects that only arise in ionic compounds, there is a significant distinction between them. The following table lists the differences between the Schottky and Frenkel defects.
| Feature | Frenkel Defect | Schottky Defect |
| Nature of Defect | Involves the displacement of an ion from its normal lattice position to an interstitial site, forming a vacancy and an interstitial defect. | Involves the vacancy formation of both cations and anions, creating vacancies in the lattice without any ions moving to interstitial sites. |
| Type of Ions Involved | Only one type of ion (typically the cation) is displaced from its lattice position. | Both cation and anion are missing from the lattice, creating an equal number of vacancies. |
| Effect on Crystal | One vacancy and one interstitial defect are formed. The overall charge neutrality is maintained. | Two vacancies are created, one for the cation and one for the anion, maintaining charge neutrality. |
| Example | Common in crystals with small cations like AgCl and ZnS. | Common in highly symmetric ionic crystals like NaCl, KCl, and CsCl. |
| Impact on Density | The overall density remains nearly unchanged because the ion displaced to the interstitial site does not affect the overall lattice size significantly. | The overall density decreases because both cation and anion are missing from the lattice, leading to a loss of mass in the crystal. |
| Charge Neutrality | Maintains electrical neutrality in the crystal because only one ion (cation) is displaced, and the vacancy does not disrupt charge balance | Maintains electrical neutrality because equal numbers of cations and anions are missing. |
| Formation Temperature | Typically occurs at high temperatures where cations gain enough energy to migrate to interstitial sites. | Occurs at high temperatures where sufficient energy is available to remove both cations and anions from the lattice. |
| Common in | Crystals with small cations that can easily fit into interstitial spaces, like silver halides (AgCl) and zinc sulfide (ZnS). | Crystals with high symmetry and closely packed ions, like NaCl, KCl, and CsCl. |
Points To Remember About The Schottky Defect
The Schottky defect is a point defect in which both cation and anion are missing in equal amounts from the lattice site. NaCl, CaCl, and other salts are examples.
Walter H. Schottky, a German physicist, discovered the Schottky Defect, often known as the small shot effect.
When heat is applied to crystals of ionic substances, Schottky defects form.
Only ionic substances have the Schottky and Frenkel defects, which are point defects. When both cation and anion depart their lattice positions and generate a pair of Vacancy Defects, the Schottky defect occurs. When an atom (particularly a cation) departs its initial lattice position and occupies an interstitial space, the Frenkel Defect occurs.
Some Solved Examples
Question.1 Which of the following have Schottky defect ?
1) ZnS
2) NaCl
3) CuBr
4) AgCl
Solution:
As we learnt in
$\%$ of missing unit $=\left(\frac{d_{\text {theoretical }}-d_{\text {experimental }}}{d_{\text {theoretical }}}\right) \times 100$
- wherein
This defect is common in the compound having a high coordination number and almost equal size of cation and anion.
ex: $\mathrm{NaCl}, \mathrm{KCl}, {\mathrm{CsCl}}, {\mathrm{AgBr}}$ etc
Crystal with higher coordination numbers are suffering from ${\text { Schottky's defect. } \mathrm{NaCl}}$ has a high coordination number i.e. 6:6.
Hence, the answer is the option (2).
Question.2 Which of the following ionic solids exhibits Frenkel defect?
1) LiF (correct)
2) NaCl
3) CaO
4) KBr
Solution:
The correct answer is option (a)LiF. LiF also known as lithium fluoride, exhibits Frenkel defect. Frenkel defect is a type of point defect in ionic solids where a cation is displaced from its regular lattice site to an interstitial site. LiF is an ionic solid in which cations are small in size and can easily move to an interstitial site, leaving behind a vacancy at its original lattice site. NaCl (sodium chloride), CaO (calcium oxide), and K Br (potassium bromide) do not exhibit Frenkel defect as their cations are larger in size and not as easily movable. Hence, the correct answer is option (a) LiF
Hence, the answer is the option (1).
Question.3 A crystal is doped with $10^{22}$ boron atoms $/ \mathrm{m}^3$. If the intrinsic carrier concentration is $5 \times 10^{16} \mathrm{~m}^{-3}$ at room temperature, what is the excess carrier concentration?
1) (correct) $8.5 \times 10^{12} \mathrm{~m}^{-3}$
2) $2.5 \times 10^7 \mathrm{~m}^{-3}$
3) $5 \times 10^7 \mathrm{~m}^{-3}$
4) $8.5 \times 10^{16} \mathrm{~m}^{-3}$
Solution:
The excess carrier concentration can be calculated as $\left(n_d-n_i\right)=\left(10^{22} \mathrm{~m}^{-3}-5 \times 10^{16} \mathrm{~m}^{-3}\right)=8.5 \times 10^{12} \mathrm{~m}^{-3}$ where $n_d$ is the donor concentration and $n_i$ is the intrinsic carrier concentration.
Hence, the answer is the option (1).
Practice More Questions With The Link Given Below
Also read -
Frequently Asked Questions (FAQs)
(i)Schottky deficiency
(ii) The crystal's density falls
(iii) Sodium chloride (Ionic solids having approximate equal size of cations and anions)
(i)LiCl's pink colour is caused by a metal excess defect in the form of anionic vacancies, in which unpaired electrons occupy the anionic sites (F-centres).
(ii) Schottky's defect is the stoichiometric defect seen in NaCl.
When magnetic moments are aligned in parallel and antiparallel directions, ferrimagnetism is found.
In the Schottky defect, ions are evacuated from the lattice, lowering the crystal's density.
(I) The density of the lattice drops in the Schottky defect, but the density of the lattice remains constant in the Frenkel defect.
(ii) In both Frenkel and Schottky faults, the electrical conductivity increases.
(i) The Frenkel defect is absent in pure alkali metal halides because the ions are too big to occupy the interstitial sites.
(ii) When ZnO is heated, oxygen is lost because Zn2+ ions are in the interstitial sites, and electrons are in neighbouring interstitial sites to preserve electrical neutrality. As a result, there is an excess of metal imperfection. Because of the presence of free electrons in the interstitial sites, it turns yellow.
(iii) Adding CaCl2 to AgCl causes an impurity fault. To preserve electrical conductivity, one Ca2+ ion will be added in place of two Ag+ ions. Ca2+ ion will occupy one of the Ag+ sites, while the other will be left vacant. As a result, a vacancy is generated, much like the Schottky defect.
NaCl, CsCl, and Kcl are among examples.
