Chromic Acid (H2CrO4) - Structure, Preparation, Properties, Uses, FAQs
Chromic acid is an oxide with chemical formula H2CrO4. Another name of chromic acid is Tetraoxochromic acid or Chromic(VI) acid. This article covers structure ,preparation ,properties and some uses of chromic acid. Chromic acid has a +6 (or VI), often known as hexavalent chromium oxidisation state. Chromium can shows a number of oxidation state,+6 is the highest among them.
This Story also Contains
- Chromic acid formula
- Structure of chromic acid
- Preparation of chromic acid
- Properties of chromic acid
- Uses of chromic acid
Chromic acid formula
Chromic acid formula is H2CrO4
Structure of chromic acid
chromic acid structure is shown below.
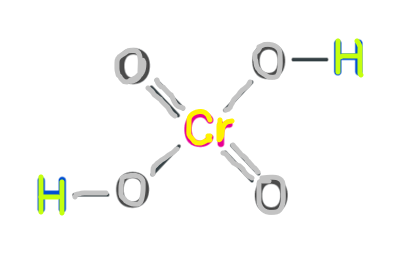
Molecular chromic acid
H2CrO4 is the molecular chromic acid Molecular chromic acid has many common things to do with sulphuric acid, H2SO4 Molecular chromic acid. It is only possible to classify sulfuric acid as part of the list of 7 strong acids. The first proton is more easily lost due to the laws related to the concept of "first order ionisation energy." It is quite similar to deprotonation of sulfuric acid. As there are more than one proton in the process of versatile acid base titrations (particularly when the acid is the beginning material and the base is the titrant), protons can leave one acid at a time.
The anhydride of molecular chromic acid is chromium trioxide. It is a Lewis acid and can react in a non-aqueous media like dichloromethane with a lewis base, such pyridine (Collins reagent).
Dichromic acid
H2Cr2O7 is the dichromic acid. Dichromic acid, is the completely protonated form of the dichromate ion. Dichromic acid can also get when you interact with aldehyde or ketone by adding chromium trioxide to molecular chromic acid. Dichromic acid is the same as aldehyde or ketone when it is reacting. However, the difference is that a secondary ketone is only oxidised by a ketone and dichromic acid only oxidises the aldehyde. It is likely present with mixed chromosulfuric acid in chromic acid cleaning solutions.
Also read -
Preparation of chromic acid
In chromic acid preparation a mixture formed by the addition of concentrated sulfuric acid to a dichromate is commonly referred to as chromic acid, which can contain a range of chemicals including solid chromium trioxide.
Properties of chromic acid
Molecular weight of chromic acid is 118.008 g/mol. The melting point of chromic acid is 197 °C Chromic acid has a boiling point of 250 °C .Chromic acid has a density of 1.201 g/cm3
Uses of chromic acid
Chromic acid uses are given below.
- Chromic acid can be used in chromium plating in the role of an intermediate
- Chromic acid is used in glasses especially in coloured glass and ceramic glass
- Chromosulfuric acid and sulfochromic mixture is a strong oxidising agent for glass cleaning in the laboratory.
- Chromic acid can shine raw metal and is therefore utilised in the tool repair sector.
- Chromic acid was used in hair colouring in 1940.
The dichromic acid H2Cr2O7 is the protonated form of the dichromate ion and can also be obtain by adding chromium trioxide to dichromic acid. It can act in the same exact way when interacting with aldehyde or ketone. The warning to this argument, is that only a ketone oxidises a secondary ketone and dichromic acid oxidises aldehyde exclusively. The aldehyde would be oxidised to a ketone for the first stage of the process and oxidised to a carboxylic acid again, subject to no significant steric obstacle to this reaction.
Chromic acid may oxidise various types of organic molecules and for this reagent a number of variants have been produced. Chromic acid is known as the Jones reagent in the aqueous sulphuric acid and acetone, which, while seldom impacting unsaturated bonds, oxidises primary and secondary alcohols, respectively, in carbonic acids and ketones.
Pyridine Chloride is produced by chromium trioxide and by pyridine chloride. This reaction transforms primary alcohols to the appropriate aldehydes (R-CHO).
Chromic acid test
In chromic acid test Jones reactant is used to oxidize aldehydes and alcohols. Jones reactant reduces chromic acid resulting in change of colour.
Related Topics, |
Reaction
Chromic acid can oxidise various organic molecules and numerous modifications have been made in this reagent.
- The Jones Reagent, which oxidises primary and secondary alcohol to carboxylic acids and ketones, while seldom damaging unsaturated bonds, is chromic acid in aqueous sulfuric acid and acetone
- Chromium trioxide and pyridinium chloride are produced with pyridinium chlorochromate. This reagent transforms primary alcohols into the relevant aldehydes (R–CHO)
- A chromium trioxide and pyridine adduct utilised for various oxidations.
- Chromyl chloride, the chemical molecule produced from chromic acid is well-determined.
Oxidation of phenol with chromic acid
Benzoquinone is generated via oxidation of phenol with chromic acid.
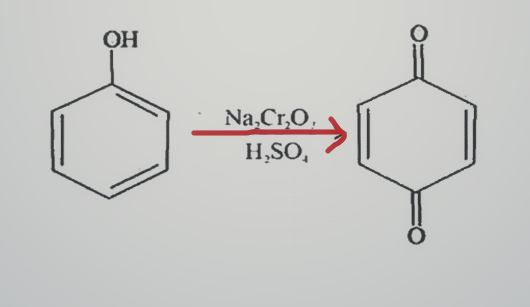
Phenol oxidation with an acidified sodium dichromate solution will be achieved in the first step. The acidified solution signifies that an acid such as sulphuric acid is present. Now the reaction is further take place to produce benzoquinone, which is a conjugated diketon.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 8 The d and f block elements
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 8 The d and f block elements
NCERT notes Class 12 Chemistry Chapter 8 The d and f block elements
Health problems
There are many hazardous and carcinogenic toxicities associated with hexavalent substances such as chromium trioxide, chrome acids, and chlorochromate. Therefore, only the aircraft industry and not other industry scales uses chromic acid oxidation.
Alternative reagents
Chromic acid is one of various reagents, including several catalytic in the oxidation of alcohols or aldehydes into carboxylic acids. Nickel (II) salts, for example, catalyse bleach oxidations (hypochlorite). Aldehydes are oxidised to carboxylic acids rather easily, and weak oxidants are plenty. For this reason, silver(I) compounds were utilized. Each oxidant has its own pros and cons. Electrochemical oxidation is often possible rather than employing chemical oxidants.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
