Colligative Properties Relative Lowering of Vapour Pressure
Have you ever wondered why adding a pinch of salt or sugar to water makes it boil at a higher temperature? Why does the vapour pressure of a solvent drop when a non-volatile solute is dissolved in it? You will get this answers by reading this article on relative lowering of vapour pressure. It is a colligative property of solutions. It is defined as the decrease in vapour pressure of a solvent when a non-volatile solute is dissolved in it, relative to the vapour pressure of the pure solvent.
This Story also Contains
- Relative Lowering of Vapour Pressure
- Raoult's Law
- Determination of Molecular Mass Using Relative Vapor Pressure Lowering Colligative Properties
- Elevation in Boiling Point
- Some Solved Examples

Relative Lowering of Vapour Pressure
The features of a Solution determined by the ratio of the number of solute particles and solvent molecules in a solution are referred to as colligative properties. A solution's colligative qualities include a relative decrease in vapour pressure, an increase in boiling point, a decrease in freezing point, and a decrease in osmotic pressure.
For all mass ratios of solute and solvent, all colligative qualities are inversely proportional to the molar mass of the solute.
Non-volatile chemistry is the inability of solute to evaporate to form gas.
.png)
Non-volatile solute or non-volatile liquid solutions exhibit the following Colligative properties:
- Relative decrease in the solvent's vapour pressure
- Its freezing point has dropped.
- A rise in the boiling point
- The solution's osmotic pressure.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Relative Lowering of Vapour Pressure
The pressure exerted by vapours over a liquid under equilibrium conditions at a certain temperature is referred to as vapour pressure. Let us now consider a pure liquid, the surface of which is occupied by the liquid's molecules. Assume that a non-volatile solute has now been introduced to this pure liquid. Because the solute molecules are non-volatile, the vapour above the solution is made up entirely of solvent (pure liquid) particles. At a given temperature, the vapour pressure of the solution is observed to be decreased than that of the liquid state after the solute is added.
This decrease in vapour pressure is caused by the fact that, after the solute was added to the pure liquid (solvent), the liquid surface now contained molecules from both the pure liquid and the solute. The number of solvent molecules that escape into the vapour phase decreases, and as a result, the pressure produced by the vapour phase decreases. This is referred to as relative vapour pressure reduction. This decrease in vapour pressure is proportional to the amount of non-volatile solute added to the solution, regardless of its type, and is thus one of the colligative qualities.
Raoult's Law
Raoult developed an empirical relationship in 1886 to establish a link between the relative decrease in vapour pressure and the concentration of solutes in the solution. This is known as Raoult's Law. According to this law, the relative decrease of a dilute solution's vapour pressure is equal to the mole fraction of solute present in it.
The evaporating molecule from the surface causes the pure solvent's vapour pressure. Because of the presence of solute molecules on the surface, when a nonvolatile solute is dissolved in solution, the surface becomes blocked and no operation occurs.
Derivations
The decrease in vapour pressure caused by a nonvolatile solute prevents solvent molecules from escaping from the solution, implying that
Ps is directly proportional to N / (n+N).
Here, N denotes the number of moles of solvent and n is the number of moles of solute.
Raoult's law is expressed as a formula.
(p-ps ) / p = n / (n + N)
Determination of Molecular Mass Using Relative Vapor Pressure Lowering Colligative Properties
Relative Lowering of Vapour Pressure Formula
The relative lowering of vapour pressure is equal to the mole fraction of solute.
A nonvolatile solute's molecular mass is computed using (p-ps). Let w grams of solute dissolve in W grams of solvent, where M and m are the molecular masses of the solute and solvent, respectively.
Then, w/m the number of moles of solute (n)
(N) = W/M
(The number of moles of solvent)
Raoult's Law equation states that
(p-ps) / p = n / (n + N)
When these numbers are substituted in Raoult's Law Equation,
(p - ps)/p = (w / m)/ (w / m + W / M)
Alternatively, (p - ps) / p = wM / mW
The molecular weight of the solute is denoted by m, and the mass of the solvent is denoted by M.
|
Related Topics Link, |
For better understanding refer below
From Raoult's law, relative lowering of vapour pressure is given as
$\frac{P^0-P_s}{P^0}=x_{\text {solute }}$
$\mathrm{P}^{\circ}=$ vapour pressure of solvent
$\mathrm{P}_{\mathrm{s}}=$ vapour pressure of solution
$\mathrm{x}=$ mole fraction of solute
$\begin{aligned} & \therefore \mathrm{x}=0.0125 \\ & x=\frac{m M}{1000+m M} \\ & \text { Where } \mathrm{m}=\text { molarity } \\ & \mathrm{M}=\text { molar mass of aqueous solution } \\ & \Rightarrow 0.0125=\frac{m \times 18}{1000+m \times 18} \\ & \Rightarrow 12.5+0.25 \mathrm{~m}=18 \mathrm{~m} \\ & \Rightarrow 17.775 \mathrm{~m}=12.5 \\ & \Rightarrow m=\frac{12.5}{17.775} \\ & \therefore m=0.70\end{aligned}$
Deviations From Raoult's Law
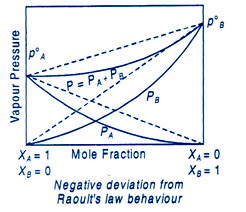
Also Read:
Elevation in Boiling Point
The vapour pressure of a liquid rises as its temperature rises. When the liquid's vapour pressure equals the air pressure (or) external pressure, the liquid begins to boil. The temperature at which the liquid's vapour pressure equals the external pressure is known as its boiling point.
A solution containing a non-volatile solute has a lower vapour pressure than pure solvent at any temperature.
Tb0 and Tb are the temperatures at which the vapour pressure of the solvent and solution equals atmospheric pressure.
$\mathrm{Tb}-\mathrm{Tb}^0=\Delta \mathrm{Tb}$
As a result, the boiling point of a solution is greater than the pure solvent's boiling point.
The boiling point of a solvent varies with the concentration of the solute in solution, but it is unaffected by the identity of the solute particles.
The boiling point rises in proportion to the concentration of the solute in the solution and is directly proportional to the molality (m) of the solute in the solution.
$\Delta \mathrm{Tb}=\mathrm{Tb}-\mathrm{Tb}^{\circ}$
$\Delta \mathrm{Tb} \alpha \mathrm{Tb}^{\circ}$
Tb -Solute Concentration
$\Delta \mathrm{Tb} ~ \alpha ~ \mathrm{~m}$ (Molarity)
$\Delta \mathrm{Tb}$ is equal to $\mathrm{K}_{\mathrm{b}} \mathrm{m}$.
Kb is the boiling point elevation constant, often known as the molal elevation constant or the ebullioscopic constant.
The molal elevation constant is defined as the increase in boiling point caused by dissolving 1 mole of a solute in 1 kilogramme of a solvent.
If w2 grams of a solute of M2 molar mass is dissolved in w1 gram of a solvent, the solution's molality (m) is,
$\mathrm{m}=\left(\mathrm{W}_2 \times 1000\right) /(\mathrm{m})\left(\mathrm{W}_1 \times \mathrm{M}_2\right)$
$\Delta \mathrm{Tb}=\left(\mathrm{W}_2 \times 1000\right) \mathrm{Kb} /\left(\mathrm{W}_1 \times \mathrm{M}_2\right)$
Regarding Rearranging:
$\mathrm{M}_2=\left(\mathrm{KbxW}_2 \times 1000\right) /\left(\mathrm{T}_{\mathrm{b}} \times \mathrm{W}_1\right)$
Also check-
Some Solved Examples
Question 1:
1) (correct) 0.6
2) 0.4
3) 0.2
4) 0.8
Solution:
$
\begin{aligned}
& \because \mathrm{P}^{\circ}-\mathrm{P} \propto \mathrm{X}_{\text {solute }} \\
& \text { and } \because 10 \propto 0.2 \\
& \therefore 20 \propto 0.4 \\
& \therefore \mathrm{X}_{\text {solvent }}=1-\mathrm{X}_{\text {solute }} \\
& \quad=1-0.4 \\
& \quad=0.6
\end{aligned}
$
Hence, the correct answer is option (1).
Question 2:
What weight of glucose must be dissolved in 100 g of water to lower the vapour pressure by 0.20 mmHg?
(Assume dilute solution is being formed)
Given: The Vapour pressure of pure water is 54.2 mmHg at room temperature. Molar mass of glucose is 180gmol-1.
1) 2.59g
2) 3.59g
3) (correct) 3.69g
4) 4.69g
Solution:
$
\begin{aligned}
& \frac{\mathrm{P}^0-\mathrm{P}_{\mathrm{s}}}{\mathrm{P}^0}=\frac{\mathrm{n}}{\mathrm{~N}}(\text { for dilute solution }) \\
& \frac{0.2}{54.2}=\frac{\mathrm{n} \times 18}{100} \\
& \mathrm{n}=\frac{100}{271 \times 18} \\
& \mathrm{w}=\frac{100 \times 180}{271 \times 18} ; \mathrm{w}=3.69 \mathrm{~g}
\end{aligned}
$
Hence, the answer is option (3).
Question 3: When a liquid that is immiscible with water was steam distilled at $952^{\circ} \mathrm{C}$ at a total pressure of 748 torr, the distillate contained 1.25 g of the liquid per gram of water. The vapour pressure of water is 648 torr at $95.2^{\circ} \mathrm{C}$, what is the molar mass of liquid?
1) $7.975 \mathrm{~g} / \mathrm{mol}$
2) $166 \mathrm{~g} / \mathrm{mol}$
3) (correct) $145.8 \mathrm{~g} / \mathrm{mol}$
4) None
Solution:
For two immiscible liquids;
$
\begin{aligned}
& P_A^0=P_{\text {total }}-P_{H_2 O}^0=748-648 \Rightarrow 100 \\
& \frac{W_A}{W_B}=\frac{P_A^0 M_A}{P_B^0 M_B} ; M_A=\frac{1.25}{1} \times \frac{648 \times 18}{100} \Rightarrow 145.8
\end{aligned}
$
Hence, the answer is option (3).
Question 4:
1) 18.0
2) 342
3) 160
4) (correct) 180
Solution:
As we learnt in
Expression of relative lowering of vapour pressure -
$\begin{aligned} & \frac{\Delta P}{P^0}=x_{\text {solute }} \\ & x_{\text {solute }}=\frac{n_{\text {solute }}}{n_{\text {solute }}+n_{\text {solvent }}}\end{aligned}$
- wherein
$\Delta P$ is lowering of v.p.
$P^0 \rightarrow$ vapour pressure of pure solvent
$x_{\text {sol ute }} \rightarrow$ mole fraction of non volatile solute
Suppose $\frac{\Lambda P}{P_0}=$ Relative lowering of vapour pressure
$$
\text { then, } \frac{\Lambda P}{P_0}=\frac{n_{\text {solute }}}{n_{\text {solute }}+n_{\text {solvent }}}
$$
$\begin{aligned} & 0.00713=\frac{71.5 / m}{71.5 / m+1000 m / 18} \\ & m=\frac{71}{0.396}=179.3 \sim 180\end{aligned}$
Hence, the correct answer is option (4)
| Vapour Pressure of Solution Containing Non-Volatile Solute Practice Question and MCQs |
| Vapour Pressure practice question and MCQs |
Frequently Asked Questions (FAQs)
A non-volatile material is one that does not easily evaporate into a gas under current conditions. Non-volatile substances do not have a considerable vapour pressure and a high boiling point than the pure solvent. Non-volatile solute includes salt and sugar. Volatile substances include alcohol, mercury, and gasoline.
In our daily lives, we use freezing point depression as a colligative trait. Many antifreezes used in automobile radiators have a lower freezing point than ordinary antifreezes, allowing automobile engines to operate in subfreezing temperatures.
Another non-colligative property is the colour of a solution. In contrast to the colourless salt and sugar solutions, the solution of CuSO4 is a vivid blue.
Non-colligative properties include freezing point, vapor pressure, boiling point.
The colligative properties of solutions are determined by the concentration of solute molecules or ions, not by the identity of the solute. Colligative properties include vapour pressure reduction, boiling point raising, freezing point depression, and osmotic pressure decrease.
With the help of a semipermeable membrane, the osmotic pressure of a solution is calculated which is a barrier with pores small enough to allow solvent molecules but not solute molecules or ions to pass through.
Solutes that are volatile. Solute Plus solvent = solution
Non-volatile substances have a low vapour pressure and a high boiling point. Non-volatile compounds have a high boiling point and a low vapour pressure.
Volatile Substance: Volatile substances easily get evaporated.
Nonvolatile Substances: They do not easily undergo evaporation.
vapour Pressure
Volatile Substance: Substances with a relatively high vapour pressure are referred to as volatile substances.
Nonvolatile Chemicals: The vapour pressure of nonvolatile substances is quite low.
Temperature at which water begins to boil
Volatile Substance: Volatile chemicals have a relatively low boiling point.
Nonvolatile Chemicals: Nonvolatile substances have a relatively high boiling point.
