Supersaturated Solution - Definition, Examples, Applications, FAQs
Have you ever watched sugar stop dissolving in your tea, no matter how much you stir, but in other cases you see extra crystals forming at the bottom after cooling? Why does a hot solution of sugar allow more crystals to dissolve than the same solution at room temperature? All these questions can be explained by studying a supersaturated solution. A supersaturated solution is a solution that contains more solute than it can normally hold at a given temperature and pressure.
This Story also Contains
- What is a Supersaturated Solution?
- Examples of Supersaturated Solution
- Supersaturation in Phase Change (Supersaturation Crystallization and Condensation)
- Applications of Supersaturated Solution
- Some Solved Examples
A homogeneous mixture in which a solute completely dissolves in a solvent is called a solution. A supersaturated solution is created when more solute is dissolved than the solvent can typically hold at a given temperature. Because the excess solute can readily crystallize out with even a small disturbance, this kind of solution is unstable. Typically, to create supersaturated solutions, the solvent is heated, excess solute is dissolved, and then it is gradually cooled. These solutions are employed in a variety of industrial applications and crystallization processes.
In the article, we cover the topic classification of supersaturated solution, which is the sub-topic of the chapter Solutions. it is important for board exams and JEE Mains Exam, NEET Exam, and other entrance exams.
What is a Supersaturated Solution?
A supersaturated solution definition in chemistry is a solution containing more than the maximum amount of solute that can dissolve in solvent at a particular given temperature. A supersaturated solution possesses an unstable state; it could be made stable by separating the excess amount of solute dissolved in the solvent.
|
Related Topics Link, |
Examples of Supersaturated Solution
Examples of supersaturated solutions include carbonated water (i.e. soda water), honey or sugar syrup used in confectionery, etc.
An example of supersaturation is shown by sodium thiosulfate $\left(\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3\right)$. It can dissolve 50 g $\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3$ per 100 g of $\mathrm{H}_2 \mathrm{O}$ at room temperature. Suppose, 70 g of $\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3$ crystal is dissolved in 100 g hot $\mathrm{H}_2 \mathrm{O}$ and the solution is then cooled to room temperature. Then the additional 20 g of $\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3$ usually does not get precipitated.
The solution thus obtained is supersaturated and it is unstable. The recrystallization in a supersaturated solution can be performed by the addition of a small crystal of solute which is called a seed crystal. This process is defined as seeding in chemistry.
The nucleation site is provided by the seed crystal on which the extra dissolved crystals can begin to grow. The supersaturated solution of $\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3$ can be seeded by the addition of a $\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3$ crystal, in which the excess salt suddenly crystallizes and heat is liberated. After the crystals are settled and the temperature has cooled back to room temperature (25°C), the solution found above the crystal is saturated and it contains 50 g $\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3$.
Supersaturation in Phase Change (Supersaturation Crystallization and Condensation)
- In each system, the physical and chemical processes of the vapor melt or the solution phase occurs by the formation of three-dimensional nuclei of a new phase and take place only during the supersaturated medium.
- The nuclei’s production is associated with a change in the free energy of the system. In the case of a homogeneous system, the new phase of the nuclei is not produced as soon as the system becomes supersaturated though thermodynamically, such a situation becomes possible.
- The system is found to be in a metastable equilibrium state, and it can remain in the same state without attaining the least or minimum free energy corresponding to the equilibrium state.
- In other words, in such cases, the nucleation of a new phase sets in after a time period, where the value depends on factors such as the pressure and temperature of the system, the presence of chemical phases varies from the increasing supersaturation level, and nucleating phase facilitates the nucleation process of the new phase.
- However, there is always a level of supersaturation when the new phase is instantaneously nucleated. It is referred to as the new phase precipitates.
- Such a supersaturation level signifies the upper limit of the metastable equilibrium state and describes the metastable width.
Also read -
Applications of Supersaturated Solution
- Supersaturation have practical applications in the field of pharmaceuticals. By preparing a supersaturated solution of a particular drug, it can be ingested in liquid form. The precipitation can be prevented by adding precipitation inhibitors. Drugs in such states are termed "supersaturating drug delivery services," or "SDDS." Oral consumption of a drug in this form is easy and helps in the measurement of very precise dosages.
- Marine ecologists use supersaturated solutions’ identification as a tool for the study of the activity of organisms and populations.
- Supersaturation is an important factor in the design of steam turbines.
- The study of supersaturation is also important for atmospheric studies. Actually, supersaturation of water is very common in the upper troposphere. This can be found using satellite data from the Atmospheric Infrared Sounder.
Also, check-
Some Solved Examples
Question 1: A beaker contains a solution of the substance ‘A’. Precipitation of substance ‘A’ takes place when a small amount of ‘A’ is added to the solution. The solution is _________.
1) Saturated
2) (correct) Supersaturated
3) Unsaturated
4) Concentrated
Solution:
Saturated, unsaturated, and supersaturated are terms used to describe the amount of solute that can be dissolved in a solvent at a given temperature:
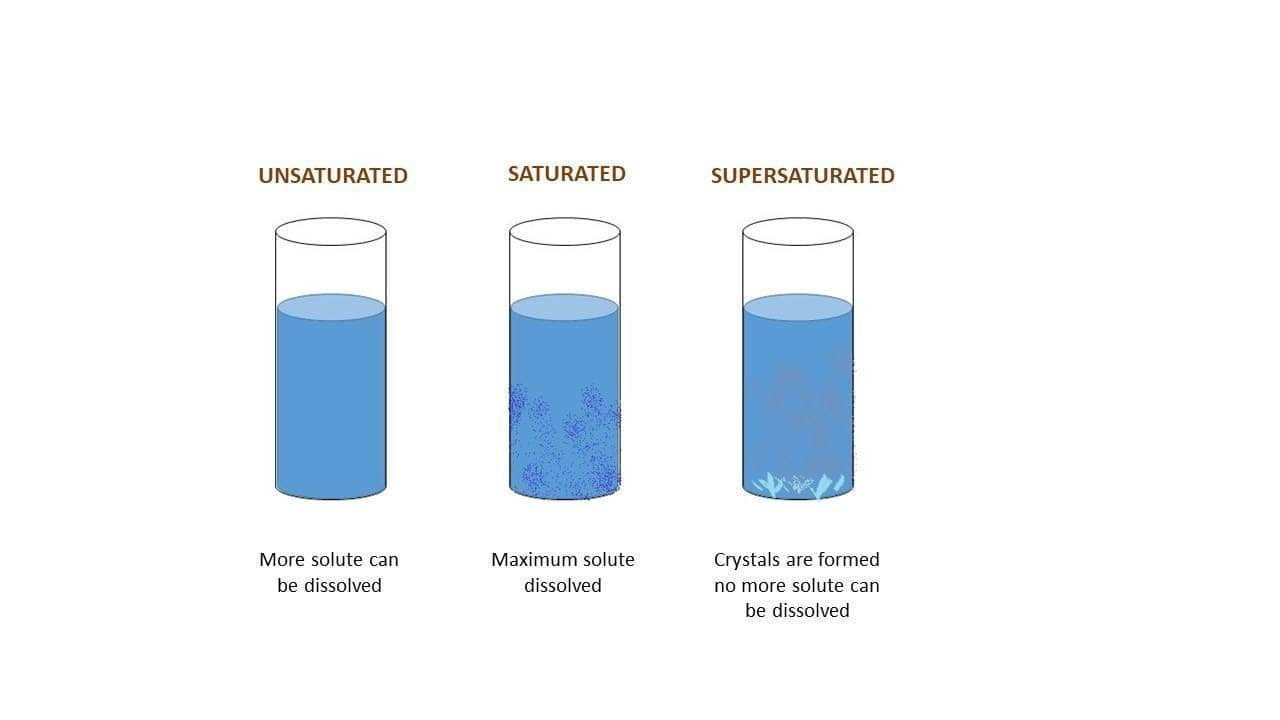
Saturated
A solution that contains the maximum amount of solute that can dissolve at a given temperature. If more solute is added, it will not dissolve and will settle at the bottom of the container as an undissolved solid.
Unsaturated
A solution that contains less than the maximum amount of solute that can dissolve at a given temperature. More solute can be dissolved in an unsaturated solution.
Supersaturated
A solution that contains more solute than the maximum amount that can dissolve at a given temperature. Supersaturated solutions are unstable and the excess solute will usually crystallize, especially if disturbed.
Adding a small amount of solute to an already saturated solution will make the solution supersaturated and will lead to the precipitation of the solute.
Hence, the answer is option (2).
Question 2: On dissolving sugar in water at room temperature solution feels cool to the touch. Under which of the following cases dissolution of sugar be most rapid?
1) Sugar crystals in cold water.
2) Sugar crystals in hot water.
3) Powdered sugar in cold water.
4) (correct) Powdered sugar in hot water.
Solution:
The solution is a homogeneous mixture of two or more chemically non-reacting substances whose composition can be varied within certain limits. A solution that contains only two components is called a binary solution. The component that has the same physical state as the solution is the solvent. In case both the components have the same physical state, then the component that is present in a larger amount is called the solvent, and the other present in a smaller amount is called the solute.
The process by which a solvent and a solute form a solution is called dissolution. When you enjoy salad dressing made with oil and water, it has to be shaken to mix the two. When this occurs, you are eating a (short-lived) dissolution on your salad.
Dissolution is an endothermic process, which is why the solution is cool to the touch. Also, powdered sugars have a higher surface area than sugar crystals, which further promotes dissolution.
Hence, the answer is option (4).
Question 3: Select the correct statement out of the following regarding a binary solution
1) A component that is present in excess is a solute
2) (correct) Component that is present in excess is solvent
3) In a solution, the physical state of the solute is retained.
4) In a solution, the physical state of the solvent is not retained.
Solution:
In a solution, the species that is present in the same state as the solution is the solvent. In case both species are in the same physical state, then the species present in excess is the solvent.
Hence, the answer is option (2).
Practice More Questions With The Link Given Below
Frequently Asked Questions (FAQs)
It is used to purify substances and produce pure crystals in industries like sugar refining, salt production, and pharmaceuticals.
A supersaturated solution contains more solute than normally possible at a given temperature. It is formed by dissolving a solute at a high temperature and then cooling it slowly without disturbing.
Because any disturbance, like adding a seed crystal or shaking, can cause the excess solute to quickly crystallize out.
Supersaturation provides the necessary condition for crystallization; when the solution becomes unstable, the excess solute forms solid crystals.
Condensation converts a gas into a liquid, while crystallization forms solid crystals from a liquid solution.
