Aluminium Sulfate - Formula, Preparation, N-factor, Uses, FAQs
A white crystalline solid, which is highly soluble in water and chemically known as $\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$ is Aluminium Sulfate, also known as alum. Aluminium Sulfate is widely used in commercial and industrial processes. You must be aware that water, before reaching our houses, is cleaned and purified by municipal water treatment facilities, and these treatment facilities widely use Aluminium sulfate as a coagulating agent that binds with dirt and other impurities, leading to the formation of large flocs which can be filtered easily. Apart from water treatment plants, this compound is used in the paper and textile dyeing industries. This compound is acidic, and due to this property, it is also used as a pH regulator in some processes. This compound is inexpensive and can be easily accessed.
This Story also Contains
- Formula and Structure of Aluminium Sulfate
- Preparation Of Aluminium Sulfate
- Properties Of Aluminium Sulfate
- How to Derive the Formula of Aluminium Sulfate
- N factor of $\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$ and Equivalent Weight
- Uses of Aluminium Sulfate
- Some Solved Examples

In this article, we cover the concept of Aluminium Sulfate, which falls under the chapter P-block in Group 13, which is important for boards and JEE Mains Exam and NEET entrance exam. Aluminium Sulfate is odourless and has a sweet taste. It is a water-soluble chemical compound, but is insoluble in ethanol. Aluminium Sulfate emits lethal fumes of sulfur oxide after undergoing the decomposition process.
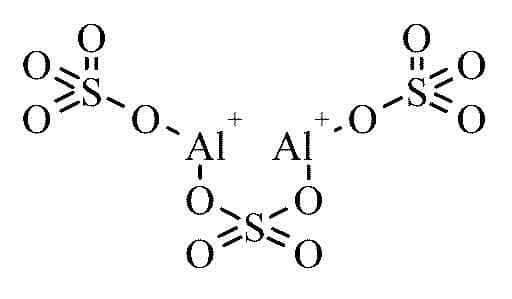
Formula and Structure of Aluminium Sulfate
The chemical formula for aluminium sulfate can be represented as - $\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$
Dialuminium Trisulfate or Filter alum are some of the other terms used to address aluminium sulphate.
The molar mass of Aluminium Sulfate is 342.15 g/cm3.
Preparation Of Aluminium Sulfate
Aluminium Sulfate can be prepared in the laboratory by the following method
-
Aluminium Sulfate is prepared by the addition of aluminium hydroxide Al(OH)3, to sulphuric acid $\mathrm{H}_2 \mathrm{SO}_4$.
$2 \mathrm{Al}(\mathrm{OH})_3+3 \mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{Al}_2\left(\mathrm{SO}_4\right)_3+6 \mathrm{H}_2 \mathrm{O}$
It can also be prepared by heating aluminium in a solution of sulfuric acid.
$2 \mathrm{Al}+3 \mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{Al}_2\left(\mathrm{SO}_4\right)_3+3 \mathrm{H}_2$
-
Similarly, $\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$ is also manufactured from alum schists, clays or bauxite and cryolite, respectively.
Alum schists comprise iron pyrite, aluminium silicate, and several bituminous elements. They undergo the roasting process, where sulphuric acid is formed and made to act on the clay to form aluminium sulphate. A comparable set of conditions is produced during the weathering. process; being another process followed for the production of aluminium sulphate.
The mass retrieved is now systematically removed with water and an aluminium sulfate solution of the required specific gravity. This solution is made to stand for some duration of time to separate. Calcium and basic iron(III) sulfate are formed. It is then evaporated until iron (II) sulfate crystallises out on chilling. It is then drawn off and evaporated until it gains the required specific gravity and is allowed to stand for some time, followed by decantation.
-
The material is calcinated lightly and then blended with sulphuric acid and water, and heated progressively to boiling. If concentrated acid is utilised then no external warmth or heat is required as the formation of aluminium sulfate is an exothermic reaction. The clear solution is extracted after being allowed to stand for a specific period of time. In this way, aluminium sulfate is extracted from clay or bauxite.
-
Calcium carbonate is blended with cryolite ore and heated, resulting in the formation of sodium aluminate. It is then again heated and precipitated by using sodium bicarbonate or by passing a current of carbon dioxide through the solution. The precipitate is then dissolved in a sulphuric acid solution. In this way, aluminium sulfate is manufactured using cryolite ore.
Also Read :
Properties Of Aluminium Sulfate
| Molecular Weight/Molar Mass | 342.15g/mol |
| Density | 2.672 g/cm3 |
| Boiling Point | 214° F/ 101.11°C |
| Melting Point | 770 °C |
How to Derive the Formula of Aluminium Sulfate
The cation and the anion in the aluminium sulfate molecule are (Al3+) and ($\mathrm{SO}^{2-}{ }_4$), respectively.
The valency of aluminium is 3, and hence it forms a trication: (Al3+)
The valency of the sulfate ion is 2, and so it forms a
Dianion: ($\mathrm{SO}^{2-}{ }_4$)
The ions combine in a way to form an electrically neutral salt.
By cross-multiplying their valencies, we get –
$\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$
The combination of ions takes place in such a way that they form an electrically neutral salt.
N factor of $\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$ and Equivalent Weight
N factor is the capability of accepting or releasing electrons. This ability is reflected by a change in the oxidation state of the species.
Hence, the n factor for salts can be simply defined as the multiplication of the charges of ions present in the salt.
Equivalent weight is defined as the ratio of molecular mass of the charged ions and charges on ionic species (n factor) i.e., the ratio of molecular weight of salt to the n factor.
How to find n factor of $\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$:
Let us find the n factor of $\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$
$\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$ compound consists of aluminium and sulfate ions having charges +3 and -2, respectively.
These ions are symbolised as – $\mathrm{Al}^{3+}$ and $\mathrm{SO}^{2-}{ }_4$.
By multiplying the charges present in the ions, we get-
3 × 2 = 6
And so, the n factor for $\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$ is 6.
Equivalent weight can be given as = M/n factor
= M/6
Related Topics:
Uses of Aluminium Sulfate
-
Aluminium sulfate functions as a mordant in the dyeing industry. It assists the dye in printing on paper or a piece of fabric.
-
Aluminium Sulphate's major use in the industrial sector is as a coagulant in water treatment plants. Aluminium Sulfate, when mixed with water, results in the formation of several forms having varied ionising levels capable of attracting contaminants present in water and precipitating them out.
-
Aluminium sulfate finds its application in paper manufacturing industries as well. It is used to eliminate unwanted foreign particles along with water. It also helps in adhering to materials and neutralising the charges when used in the pulp itself.
-
Aluminium Sulfate helps regulate algal growth in water bodies. Aluminium Sulfate, being an acidic compound, is often used by gardeners to adjust the soil pH.
-
It is also used as an accelerator and waterproofing agent in the concrete business.
-
One can find its application as a firefighting foam and fireproofing agent as well.
Also, check-
Some Solved Examples
Question 1: The aqueous solution of $\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$ is:
A. Neutral
B. Acidic
C. Basic
D. Amphoteric
Solution:
$\mathrm{Al}^{3+}\left(\right.$ from a weak base $\left.\mathrm{Al}(\mathrm{OH})_3\right)$ hydrolyzes and releases $\mathrm{H}^{+} \rightarrow$ solution becomes acidic.
Hence, the correct answer is option (A)
Question 2: Which ions are formed when $\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$ is dissolved in water?
A. $2 \mathrm{Al}^{3+}+3 \mathrm{SO}_4{ }^{2-}$
B. $2 \mathrm{Al}^{2+}+3 \mathrm{SO}_4^{-}$
C. $3 \mathrm{Al}^{+}+2 \mathrm{SO}_4{ }^{3-}$
D. $\mathrm{Al}^{4+}+\mathrm{SO}_4{ }^{4-}$
Solution:
The cation and the anion in the aluminium sulfate molecule are (Al3+) and ($\mathrm{SO}^{2-}{ }_4$), respectively.
The valency of aluminium is 3, and hence it forms a trication: (Al3+)
The valency of the sulfate ion is 2, and so it forms a
Dianion: ($\mathrm{SO}^{2-}{ }_4$)
Hence , the correct answer is option (A)
Question 3: The first stage hydrolysis product of $\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$ in water is:
A. $\mathrm{Al}(\mathrm{OH})_3$
B. $\mathrm{AlOHSO}_4$
C. $\mathrm{Al}_2 \mathrm{O}_3$
D. $\mathrm{Al}_2\left(\mathrm{SO}_3\right)_3$
Solution:
When aluminium sulfate is dissolved in water, the $\mathrm{Al}^{3+}$ ions undergo hydrolysis.
But complete hydrolysis forming $\mathrm{Al}(\mathrm{OH})_3$ does not occur immediately.
Instead, in the first stage of hydrolysis, a basic salt is formed:
$\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3+\mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{AlOHSO}_4+\mathrm{H}_2 \mathrm{SO}_4$
This product is called basic aluminium sulfate.
Hence , the correct answer is option (B)
Frequently Asked Questions (FAQs)
There are several methods used in the preparation of Aluminium Sulfate, but the most widely used one is the reaction of Aluminium hydroxide with Sulfuric acid.
The reaction is represented as:
2Al(OH)3 + 3H2SO4 $\rightarrow$ Al2(SO4) + 6H2O
Aluminium sulfate is represented chemically as Al2(SO4)3. This compound consists of two ions of Aluminium and three ions of (SO4). Aluminium sulfate is a fundamental chemical used widely in water purification, paper, and textile dying industries.
N-factor of Al2(SO4)3 is 6. N-factor plays a very important role in stoichiometric calculations like titration reactions, as it helps in determining the number of equivalents in a reaction.
Aluminium Sulfate is used in various industries:
- In the water treatment industry as a water purifier.
- In the textile industry acts as a mordant to fix dyes on fabric
- It is used in the paper industry as a sizing agent.
Aluminium sulfate is safe, but there are certain precautions that we can follow:
- Inhalation of aluminium sulfate dust and fumes should be avoided.
- It can cause skin irritation upon contact
So to protect ourselves from these side effects, we can use a mask, gloves, and goggles when handling this compound. This compound should be stored in a dry and cool place.
