Daniell Cell - Explanation, Diagram, Functions with FAQs
Have you ever wondered how a simple chemical reaction can generate electrical energy? The answer is a Galvanic cell, specifically a Daniell cell provides us a perfect example of this. Daniell cell not only revolutionised the way we generate electricity but also laid the groundwork for understanding modern battery technology. Daniel's cell generates electrical energy through a redox (reduction-oxidation) reaction. It was invented by John Daniell in 1836 and is one of the simplest examples of a working battery.
This Story also Contains
- What is a Daniell Cell?
- Explanation Of the Daniell Cell
- Working Of the Daniell Cell
- Salt bridge
- Some Solved Examples
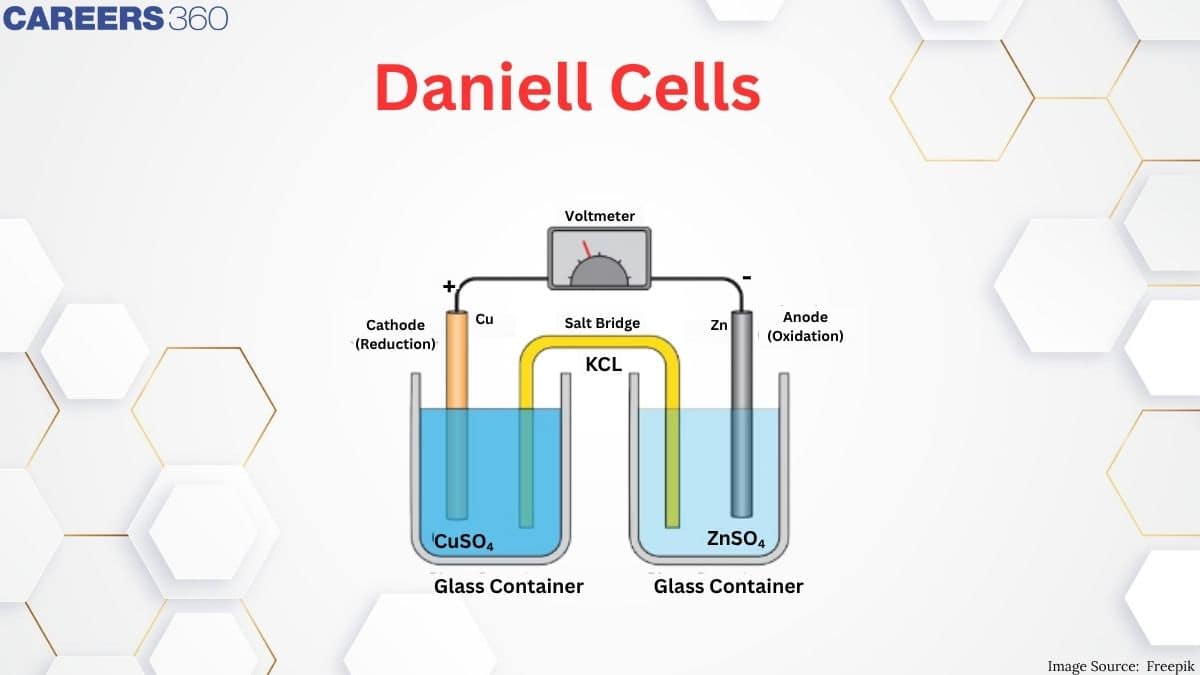
What is a Daniell Cell?
The greatest example of a galvanic cell that turns chemical energy into electrical energy is a Daniell cell.
The Daniell cell is made up of two electrodes made of different metals, Zn and Cu, which are in contact with a solution of their respective ions, zinc sulphate and copper sulphate.
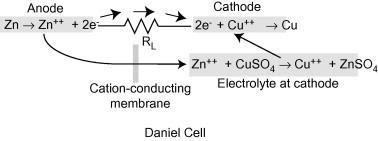
Explanation Of the Daniell Cell
A conventional galvanic cell is meant to generate an electric current by using the spontaneous redox reaction between zinc and cupric ions.
A copper vessel makes up this cell. In this case, a saturated CuSO4 solution is used as a depolariser and diluent. Fill with H2SO4, which works as an electrolyte. Zn2SO4 is used to submerge a zinc rod that has been amalgamated. CuSO4 crystals are kept in touch with CuSO4 solution by a transparent layer just below the upper surface of copper vessels, ensuring that the solution is always saturated.
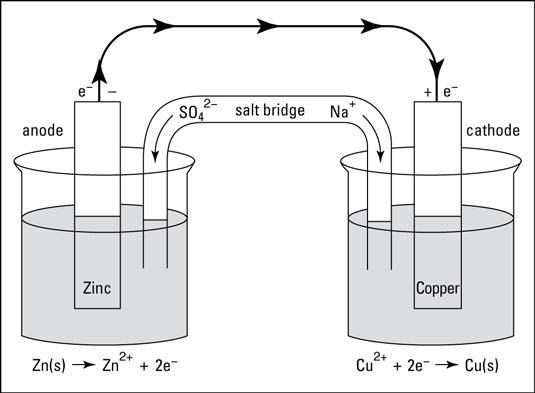
Working Of the Daniell Cell
Daniel's cell working is discussed below.
$\mathrm{Zn}(\mathrm{s})+\mathrm{Cu}^{2+}(\mathrm{aq}) \rightarrow \mathrm{Zn}^{2+}(\mathrm{aq})+\mathrm{Cu}(\mathrm{s})$
In a Daniell cell, electrons flow through an external circuit from the zinc electrode to copper electrode to copper electrode, while metal ions move from one half cell to the other via the salt bridge.
Through an external circuit, current flows from the copper electrode to the zinc electrode, which is the cathode, to the anode.
A voltaic cell can be reversible or irreversible, whereas a Daniell cell is reversible.
Cell Reaction
Chemical Reaction of Daniell Cells
$\mathrm{Zn}+\mathrm{Cu}^{2+} \rightleftharpoons \mathrm{Zn}^{2+}+\mathrm{Cu}$
Daniel's cell representation
$\mathrm{Zn} / \mathrm{Zn}^{2+} \| \mathrm{Cu}^{2+} / \mathrm{Cu}$
The above reaction can be divided into two parts:
Half-cell anode reaction
$\mathrm{Zn} \rightarrow \mathrm{Zn}^{2+}+2 \mathrm{e}^{-}$
Zinc metal is oxidised once two electrons are liberated.
Reduction half-cell reaction
$\mathrm{Cu}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}$
Copper metal is formed when copper ions are reduced to copper metal
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 3 Electrochemistry
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 3 Electrochemistry
- NCERT notes Class 12 Chemistry Chapter 3 Electrochemistry
Daniell Cell Construction
It's made up of a copper container filled with a concentrated copper sulphate solution. One porous cylindrical pot filled with diluted sulphuric acid is immersed in the concentrated copper sulphate solution inside the container. In a porous pot, one amalgam zinc rod is immersed in dilute sulphuric acid. Sulphuric acid in its diluted state has positive hydrogen ions and negative sulphate ions, which is a feature of dilute electrolytes.
When sulphate ions come into contact with zinc rods, they release electrons and generate zinc sulphate as a result of the oxidation reaction. As a result, the zinc rod acquires a negative charge and takes on the role of a cathode.
Positive hydrogen ions can pass through the porous wall of the pot and into the copper sulphate solution, where they combine with copper sulphate electrolyte sulphate ions to generate sulphuric acid. The positive copper ions in the copper sulphate electrolyte come into contact with the copper container's inner wall, where they are reduced and produce copper atoms, which are then deposited on the wall.
|
Related Topics, |
Daniell Cell's Operation
For a better understanding, let us go over the cell's operating concept step by step. In diluted sulfuric acid solution, there are H+ and $\mathrm{SO}_4^{--}$ ions. The H+ ions come out to the copper sulphate solution through the wall of the porous pot. The sulphate ions of diluted sulfuric acid react with the zinc rod, where Zn++ ions get attached with $\mathrm{SO}_4^{--}$ ions and form zinc sulphate (ZnSO4). During this oxidation reaction, each zinc atom leaves two electrons in the zinc rod. Hence, the zinc rod becomes negatively charged, which means it behaves as the cathode of the battery.
The hydrogen ions (H+) in the copper sulphate solution form sulfuric acid (H2SO4), and copper ions ($\mathrm{Cu}^{++}$) come to the wall of the outer copper container. By absorbing electrons from the container, copper ions deposit as copper metal on the container's wall. As a result, the copper container becomes positively charged, indicating that it serves as the Daniell Cell's anode. When an external load is connected between the central zinc rod and the peripheral copper container wall, electrons begin to flow from the zinc rod to the copper container.
Voltaic Cell Conditions
Because the external source's emf is greater than that of the voltaic cells, current can flow from the external source into the voltaic cell, reversing the cell reaction. Current flows from the voltaic cell to the external source if the emf of the voltaic cell is greater than that of the external source.
Salt bridge
Salt bridge helps to maintain the electrical neutrality in two compartments by allowing movement of anions towards the anodic compartment and cations towards the cathodic compartment. A salt bridge is usually made by filling a U-tube or a tube with a gel or agar-agar containing an inert electrolyte, typically a salt like potassium nitrate ($\mathrm{KNO}_3$) or sodium chloride (NaCl). It's a gelatin-coated glass tube containing potassium chloride or ammonium nitrate.
Function of a Salt Bridge
Between the two half-cells, a salt bridge serves as an electrical contact.
- It restricts the mechanical movement of the solution but allows ions to migrate freely, allowing an electric current to pass through the electrolyte solution. It stops charges from building up.
- The charge balance in the two half-cells is maintained with the help of a salt bridge.
- The liquid connection potential is reduced or eliminated by using a salt bridge.
Also read -
Some Solved Examples
Question 1: Which of the following is true about chemical cells?
1) Convert chemical energy to electrical energy
2) Most batteries are chemical cells
3) (correct) i and ii
4) none
Solution:
Chemical Cells - The cells in which electrical energy is produced from the energy change accompanying chemical reactions or a physical process are known as chemical cells. Chemical cells convert chemical energy to electrical energy
Hence, the answer is option (3).
Question 2: Galvanisation is applying a coating of :
1) Pb
2) Cr
3) Cu
4) (correct) Zn
Solution:
As we learned
Exceptions of transition elements -
Zn, Cd, and Hg have the following configuration
$(n-1) d^{10} n s^2$ But they don't have empty 'd' orbitals that's why they are called 'd' block elements but not transition elements.
Zinc is used to prevent rusting.
Therefore, galvanisation is the application of a coating of Zn.
Hence, the answer is option (4).
Question 3: In a Daniell cell, which of the following occurs at the anode?
A) Reduction of $\mathrm{Cu}^{2+}$ to Cu
B) (correct) Oxidation of Zn to $\mathrm{Zn}^{2+}$
C) Oxidation of Cu to $\mathrm{Cu}^{2+}$
D) Reduction of $\mathrm{Zn}^{2+}$ to Zn
Solution:
At the anode, oxidation occurs. In the Daniell cell, zinc $(\mathrm{Zn})$ is oxidized to $\mathrm{Zn}^{2+}$ ions. The reaction is:
$\mathrm{Zn}(s) \rightarrow \mathrm{Zn}^{2+}(a q)+2 e^{-}$
So, zinc metal is oxidised, losing electrons.
Hence, the correct answer is option (b)
Practice More Questions With The Link Given Below
Frequently Asked Questions (FAQs)
The Daniell cell has several advantages, including:
- It produces a stable voltage of approximately 1.1 volts, which is higher than other early primary cells, like the Voltaic cell.
- It provides a constant current output over a long period, making it suitable for various applications, such as telegraphy and electrical measurements.
- It does not produce hydrogen gas, which is a common issue in other early electrochemical cells, like the Voltaic cell.
- The zinc and copper metals used in the cell are relatively inexpensive and widely available.
Some limitations of the Daniell cell are:
- The cell has a relatively low energy density compared to modern batteries.
- The cell is bulky and requires a large amount of electrolyte, making it less portable than modern batteries.
- The porous barrier between the two solutions can become clogged over time, reducing the cell's efficiency.
- The cell is not rechargeable, and once the reactants are consumed, the cell cannot be used again.
The Daniell cell is based on the redox reaction theory. Electrons can be transmitted as useful electrical current during the reaction cycle.
Daniell Cell Process Steps
Zn(s)+Cu+2? Zn+2(aq)+Cu+2(s)
In a Daniell cell, electrons flow through an external circuit from zinc electrode to copper electrode to copper electrode, while metal ions move from one half cell to the other via the salt bridge.
Through an external circuit, current flows from the copper electrode to the zinc electrode, which is the cathode to the anode.
A voltaic cell can be reversible or irreversible, whereas a Daniell cell is reversible.
