Electroplating Process - Overview, Advantage & Disadvantage, Uses, FAQs
Have you ever wondered how ordinary metals acquire a shiny, rust-resistant surface? How does jewellery get its gold coating, or how do car parts and electronic devices gain added strength and beauty? You will get these answers by reading this article on electroplating. The process of depositing a thin layer of metal over another metal object with the help of an electric current is called electroplating. Electroplating is mostly used to modify the surface properties of an object (for example, corrosion resistance, lubricity, and abrasion resistance), but it can also be used to add thickness or create objects by electroforming.
This Story also Contains
- Electroplating
- Anode and Cathode
- How Does Electroplating Work?
- Silver Plating
- Copper Plating
- Metals Used in Electroplating
- Uses of Electroplating
- Advantages of Electroplating
- Disadvantages of Electroplating
- Some Solved Examples
In this article, we cover the concept of electroplating, which falls under the Electrochemistry chapter of class 12. This topic is important for boards and for the JEE Mains Exam, National Eligibility Entrance Test (NEET) point of view. Electroplating has advantages in artificial jewellery that shines while it is new, but then fades after a while. Electroplating brass with copper is a good example of this. Brass and copper would be placed in a suitable electrolyte solution in this case. A copper sulphide-containing solution is preferred in this case. Electrodes and a battery would then be linked to each piece of metal. When the electricity is turned on, the copper molecules gently bond to the brass, forming a thin copper coating on the brass's surface.
Electroplating
Electroplating is an electrochemical process in which a thin layer of one metal is deposited on the surface of another metal by using an electric current. This process is widely used to improve the appearance, corrosion resistance, hardness, and durability of metals.
Principle
Electroplating works on the principle of electrolysis:
- When an electric current passes through an electrolyte, metal ions move toward the cathode.
- These ions gain electrons (reduction) and get deposited as a thin metal layer.
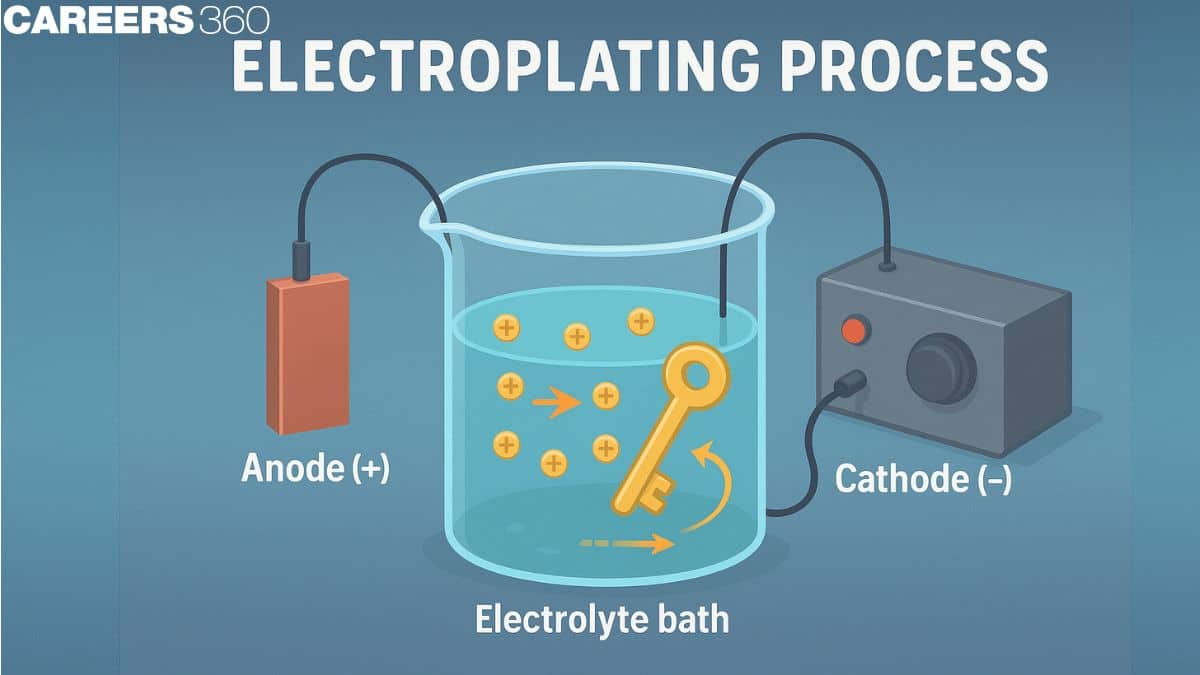
Anode and Cathode
In electroplating, the current is normally introduced from an external source. In electroplating, the anode is the positive electrode and the cathode is the negative electrode. The electrochemical reduction reaction takes place at the cathode. The electrochemical Oxidation reaction takes place at the anode. An anode and a cathode are used in the electroplating process. The metal dissolved from the anode can be plated onto the cathode in electroplating. Direct current is applied to the anode, which oxidizes and dissolves the metal atoms in the electrolyte solution. The dissolved metal ions are reduced at the cathode, and the metal is deposited on the product.
How Does Electroplating Work?
Let us use the example of a gold coating to further comprehend the concept. A layer of gold is to be electrodeposited on metallic jewelry, in this case, to improve its appearance. The gold plating is usually linked to the circuit's anode (+ve charged electrode), while the jewelry is kept at the cathode (-ve charged electrode). Both are immersed in an electrolytic bath (solution). A DC current is applied to the anode at this point, which oxidizes the gold atoms and dissolves them in the solution.
At the cathode, gold-dissolved ions are reduced and plated on the jewelry.
However, there are a number of significant parameters that determine the final plating. These are some of them:
-
The distance between the cathode and anode
-
The duration of electroplating
-
Voltage level of current
-
Chemical composition of the bath
-
Temperature of the bath
Also read -
Silver Plating
Silver plating is the process of coating an object with a thin layer of silver by electrolysis of a silver salt solution. It improve appearance (shiny finish), increase corrosion resistance, enhance electrical conductivity, and reduce cost by using less silver
Copper Plating
The object to be plated is connected to the power supply's negative terminal. The positive terminal is attached to a piece of copper. A copper sulfate solution serves as the electrolyte. Copper deposition, like silver plating, can be used to purify copper. Both electrodes are constructed of copper in this scenario. The negative electrode eventually becomes coated with pure copper as the impure positive electrode fades away, leaving the impurities behind.
Metals Used in Electroplating
There are a variety of metals that are ideal for electroplating:
1. Silver (Ag) - For ornaments, cutlery, electrical contacts
2. Gold (Au) - For jewellery, electronic components (corrosion resistance)
3. Nickel (Ni) - For corrosion resistance and decorative finish
4. Chromium (Cr) - For hardness, shine, and wear resistance (car parts, taps)
5. Copper (Cu) - As a base layer before nickel/chromium plating
6. Zinc (Zn) - To prevent rusting of iron (galvanization)
7. Tin (Sn) - For food containers, corrosion protection
8. Cadmium (Cd) - For aerospace parts (now limited due to toxicity)
Uses of Electroplating
Electroplating in Aesthetics:
Gold and silver, which are scarce and valuable metals, are common examples of this application.
Electroplating allows a small coating of these valuable metals to be deposited on a less expensive metal, giving the finished product a more appealing appearance at a reduced cost. This is one of the electroplating's most widely used commercial uses today. This method is employed in the creation of jewelry and other accessories.
Electroplating in Commercial Application:
For a smooth texture and look, automotive parts are electroplated with a thin layer of chromium. They are also done on a variety of other appliances that are customized to the buyer's specifications.
Electroplating to Prevent Rusting:
Because metals are susceptible to natural phenomena such as corrosion, a thin layer of non-corrosive metals over the corrosive one can extend the life of metals and appliances. Non-corrosive metals like copper, chromium, and nickel are commonly used to coat corrosive metals like steel and iron these days.
Electroplating in the Conduction of Electricity:
Good conductors of electricity, such as gold and silver, are employed in integrated circuits used in computers, cell phones, and other electronic devices. However, due to the expensive cost of these metals, a small amount of these costly high-quality metals can be coated as a thin layer over other metals to aid in electrical conduction.
Electroplating To Reduce Friction:
Apart from these applications, electroplating can be utilised in other areas where friction can be reduced by layering one metal over another.
Protection from Radiation:
Electroplating also helps to defend against radiation, abrasion, and a variety of other natural phenomena by adding desired characteristics to a metal surface that lacks them.
Advantages of Electroplating
Many businesses have benefited for decades from electroplating's ability to boost the durability or improve the aesthetic of plated items.
- The automotive industry is one of the most common industrial applications of electroplating. Old parts, such as bumpers, grills, and tire rims, are commonly electroplated to make them look new.
- When corrosion versus protection is a concern in the workplace, electroplating is a preferred option.
- Because of its higher conductivity, silver electroplating is frequently employed on copper or brass connectors.
- Copper electroplating has a lot of uses, including the production of electronic equipment and components, as well as goods for the aerospace and defence industries.
Disadvantages of Electroplating
The waste generated during the electroplating process is difficult to dispose of in the environment since it poses a health risk.
-
Making numerous coats of metal is a time-consuming process.
-
Electroplating requires a lot of expensive equipment.
Also, check-
Some Solved Examples
Question 1: Electroplating is based on which process?
A. Combustion
B. Electrolysis
C. Distillation
D. Sublimation
Solution:
Electroplating uses an electric current to drive a non-spontaneous redox reaction that deposits metal ions on a surface. That is the definition of electrolysis.
Hence, the correct answer is option (B)
Question 2: In electroplating, the object to be plated is connected to:
A. Anode
B. Cathode
C. Both electrodes
D. Ground terminal
Solution:
Reduction (gain of electrons) causes metal ions in solution to become metal atoms and deposit. Reduction occurs at the cathode, so the object to be plated is made the cathode.
Hence, the correct answer is option (B)
Question 3: What acts as the anode during copper electroplating?
A. Copper rod
B. Iron nail
C. Zinc rod
D. Carbon rod
Solution:
In conventional copper electroplating, the anode is a copper bar/rod. At the anode copper oxidizes dissolving as $\mathrm{Cu}^{2+}$ into the solution and replenishing $\mathrm{Cu}^{2+}$ ions:
$\text { Anode (oxidation): } \mathrm{Cu}(\mathrm{~s}) \rightarrow \mathrm{Cu}^{2+}(\mathrm{aq})+2 e^{-}$
This maintains the ion concentration so the cathode gains copper.
Hence, the correct answer is option (A)
Question 4: Which of the following reactions occurs at the cathode during electroplating?
A. Oxidation
B. Reduction
C. Substitution
D. Hydrolysis
Solution:
Metal cations gain electrons and are reduced to metal atoms at the cathode, e.g. for copper:
$\text { Cathode (reduction): } \mathrm{Cu}^{2+}(\mathrm{aq})+2 e^{-} \rightarrow \mathrm{Cu}(\mathrm{~s})$
So the correct process is reduction.
Hence, the correct answer is option (B)
Question 5: The most suitable electrolyte used for silver plating is:
A. $\mathrm{AgNO}_3$
B. AgCl
C. $\mathrm{K}\left[\mathrm{Ag}(\mathrm{CN})_2\right]$
D. $\mathrm{Ag}_2 \mathrm{SO}_4$
Solution:
Potassium silver cyanide provides controlled Ag⁺ concentration, ensuring smooth and uniform deposition.
Hence, the correct answer is option (C)
Frequently Asked Questions (FAQs)
A number of elements have an impact on this process. The electrodes' surface area, temperature, the type of metal and electrolyte employed, and the amplitude of the applied current are all elements to consider.
Methanesulphonic acid is used in the electroplating and metal finishing industries. Over the last ten years, methanesulphonic acid has gradually replaced fluoroboric acid as the preferred electrolyte for the electrodeposition of tin and tin-lead solder on electronic devices.
The process of depositing one metal over another in the presence of metal salt is known as electroplating (in an aqueous solution). In this process, the water molecule is liberated as the ultimate result. As a result, electroplating is based on the hydrolysis theory.
Most commonly for decorative purposes or to prevent corrosion of a metal. There are also specific types of electroplating such as copper plating, silver plating, and chromium plating.
Common metals used for electroplating include gold, silver, nickel, chromium, copper, and zinc. The choice of metal depends on the desired properties of the finished product.
Electroplating is used in various industries for applications such as decorative finishes (e.g., jewelry, automotive parts), corrosion resistance (e.g., galvanized steel), increasing hardness (e.g., tools), and enhancing electrical conductivity (e.g., electronic connectors).
Advantages of electroplating include improved aesthetics, enhanced resistance to corrosion and wear, increased electrical conductivity, reduced friction, and the ability to achieve precise thickness and patterns on the substrate.
