Difference Between Alkali and Base - Definition, Concept, Properties, Uses, FAQs
Difference Between Alkali and Base
It had taken centuries for the theory of acids and bases to take shape. Acids taste sour, and that's why they got their name (the Latin word acidus means sour): they're acidic. On the other hand, bases taste bitter. Arrhenius was the first to classify acids and bases scientifically. However, with time, the definition has changed in order to generalize and incorporate all the acids and bases under a single definition respectively. Alkalis are bases only but can be dissolved in the water. This article discuss about definition of alkali and bases and also the differences between alkali and base.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Bases
There are three distinct definitions for a base and every certainly considered one among them defines a base in its personal way. These three definitions for a base are as follows
Arrhenius Concept: Any compound which furnishes OH- ions in its aqueous answer is named as a base. This definition covers all of the hydroxides of alkali metals and alkaline earth metals bases which includes NaOH, Ca (OH)2 etc. This is a best definition of a base. In trendy bases are the compounds that react with acids to supply salt. This definition covers a number of the bases however leaves out many. So, different definitions of bases have been given via way of means of one-of-a-kind scientists.
Bronsted Lowry Concept: Any compound this is able to accepting a proton i.e., a H+ ion is named as a base. By this definition, even H2O can act as a base as it may gather a proton to shape hydronium ion i.e., H3O+ ion. This definition covers maximum of the bases however nevertheless leaves the various bases due to the fact now no longer all bases extract H+. AlCl3 acts as a Lewis acid however it has no H+ to supply. So, bases which react with such acids can’t be defined via way of means of this definition. So, any other definition of a base became given via way of means of Lewis.
Lewis Concept: Any compound that could donate a lone pair or can donate a couple of electrons is named as a base. For instance: NH3 is a Lewis base. It has a lone pair which it may donate. Lewis base normally extract protons and turn out to be strong which includes NH3, that acquires a proton to shape NH4+. So, in keeping with this definition any compound, ion or detail which could donate a couple of electrons to the opposite species can act as a base. It may be CuO, ZnO. It is a greater generalized definition. NaOH, and KOH supply OH- which could donate a couple of electrons. Therefore, Lewis idea covers maximum of the elements of a base.
What is Alkali?
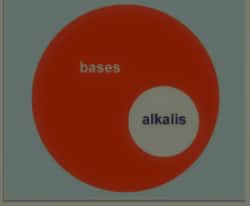
Alkalis are water soluble bases. In different words, bases which may be dissolved in water to grant OH- ions are termed as alkali. Alkalis are like a subset of bases. So, we are able to say that “All alkali are bases however all bases aren't alkali”. The time period alkali is particularly used for the hydroxides of alkali metals or alkaline earth metals as they effortlessly dissolve in water to grant OH- ions. Alkali flips pink litmus blue and are sour to taste. And, pH of a alkali answer is extra than 7.
Examples of alkali:
NaOH, KOH, Be (OH)2, Ca (OH)2
Sodium hydroxide (NaOH) – caustic soda
Potassium hydroxide (KOH) – caustic potash
Calcium hydroxide, {Ca (OH)2 } – limewater
Difference Between Alkali and Base
In general, people use those phrases interchangeably however there's a primary distinction among the . Alkali metal hydroxides and alkaline earth metal hydrides are classified as alkali metal hydroxides. They do now no longer consist of NH3. So as in line with this definition, NH3 isn't an alkali although it is a base. Similarly, there’s some other definition to it which is going this way: The bases which dissolve in water to provide OH- are alkalis. This definition additionally excludes NH3. So the distinction lies withinside the truth that alkali is sort of a subset of bases. That’s why we will say, all alkali are bases however now no longer all bases are alkali. The differences between alkali and bases include
Concept
The base is a material that, when dissolved in water, raises the concentration of OH-ions. Alkalis dissolve easily in water and give a clear solution. It's possible that the solution will have a repulsive odor. However, the amount of water, alkali, and alkali's PH value all play a role. This is one of the main differences between alkali and base.
Characteristics
Both the physical and chemical properties of alkali are the same. They are pliable and easily cut with a knife if desired. They’re made of a material with a low melting point and density. Sodium, potassium, and lithium, for example, have a density that makes them afloat in water.This is another difference between alkali and base.
Uses
Organic tannins, fluorides and other pollutants are removed from water using alkalis. They're utilized to make water's pH more alkaline. Alkali is used to keep the sewage sludge clean and to cut down on odors. To make wastewater more visible, they're employed in manufacturing and mining. This also aids in the removal of phosphate and nitrogen. When it comes to kitchens, table salt (sodium chloride) is an alkali that is used. For example, bases can be found in laxatives, as well as soaps, detergents, and other household cleaners. They are also used to neutralize acidic wastewater as a non-hazardous alkali. Antiperspirant deodorant contains them as well.
Properties
Bases have a harsh flavor and a slick, soapy feel to them. They produce water and salt molecules when they react with acids. All three of these substances can be used to make various types of bases. Alkalis are metals with a silvery hue that are highly reflective and pliable. One electron covers the outside of their shell. Cations arise when an ion is withdrawn. This is also a difference between alkali and base.
Also, students can refer,
- NCERT solutions for Class 10 Science Chapter 2 Acids, Bases and Salts
- NCERT Exemplar Class 10 Chemistry Solutions Chapter 2 Acids, Bases and Salts
NCERT notes Class 10 Chemistry Chapter 2 Acids, Bases and Salts
Dissociation in Water
When bases and water are combined, the bases breakdown to produce free hydrogen ions (OH-). Alkalis, on the other hand, react aggressively when coupled with water. As a result of the reaction, hydrogen gas and base (a very alkaline solution) are produced. This is also a difference between alkali and base.
It clear that all alkalis are bases but all bases are not alkalis.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
