Electrochemical Cell - Definition, Examples, Types, Uses, FAQs
What happens when a chemical reaction itself becomes a source of electricity? How can the spontaneous flow of electrons in a redox reaction be converted into usable electrical energy? An electrochemical cell provides the answer by allowing oxidation and reduction reactions to occur in separate half-cells, forcing electrons to travel through an external circuit. In this way, chemical energy is transformed directly into electrical energy, forming the basis of batteries, fuel cells, and many modern electrochemical devices.
This Story also Contains
- Electrochemical Cell
- Cell Representation
- Electrochemical Cell Diagram
- Half-Cells and Cell Strength
- Lower and Second Cells
- Types of Electric Cells
- Electrolytic Cell Function
- Some Solved Examples
Electrochemical Cell
An energy cell is a machine that can generate electricity from the chemical reactions that occur in it, or use the electrical energy given to it to make chemical reactions in it. The given devices are capable of converting a chemical energy into an electrical energy, or even vice versa. A common example of an electrochemical cell is a standard 1.5-volt cell used to power many electronic devices such as TV remote and clocks. Such cells are capable of producing energy from the chemical reactions that occur in them and maintain the so-called Galvanic cells or Voltaic cells. In other words, the cells that cause the chemical reaction to take place when they pass through electrical energy are called electrical cells.
Example of Electrochemical Cell
A typical example of an electrochemical cell is a standard 1.5 volt cell intended for consumer use. A battery consists of one or more cells, usually connected in parallel, series or even series pattern and parallel. We interact with electrical cells in all aspects of our daily lives from disposable AA batteries in our remote controls and lithium-ion batteries on our iPhones to the nerve cells scattered throughout our bodies. There are basically two types of electrical cells namely galvanic cells, also called Voltaic cells and electrolytic cells. Important examples of electrolysis are the decomposition of water into hydrogen and oxygen, and bauxite into aluminum and other chemicals. Electroplating (e.g., copper, silver, nickel or chromium) is performed using an electrolytic cell. Electrolysis is a process that uses direct electric current (DC).
Cell Representation
Keep in mind that normal cell power can be calculated from the E0cell probability in both the connection and the reduction response. The positive energy of the cell indicates that the reaction continues spontaneously in the direction in which the response is recorded. On the other hand, a malignant cell reaction is found automatically on the reverse side. Cell recognition is a brief description of voltaic or galvanic (automatic) cells. Response conditions (pressure, temperature, concentration, etc.), anode, cathode, and electrode components are all described in this unique shortcut. Remember that oxidation occurs in the anode and reduction occurs in the cathode.
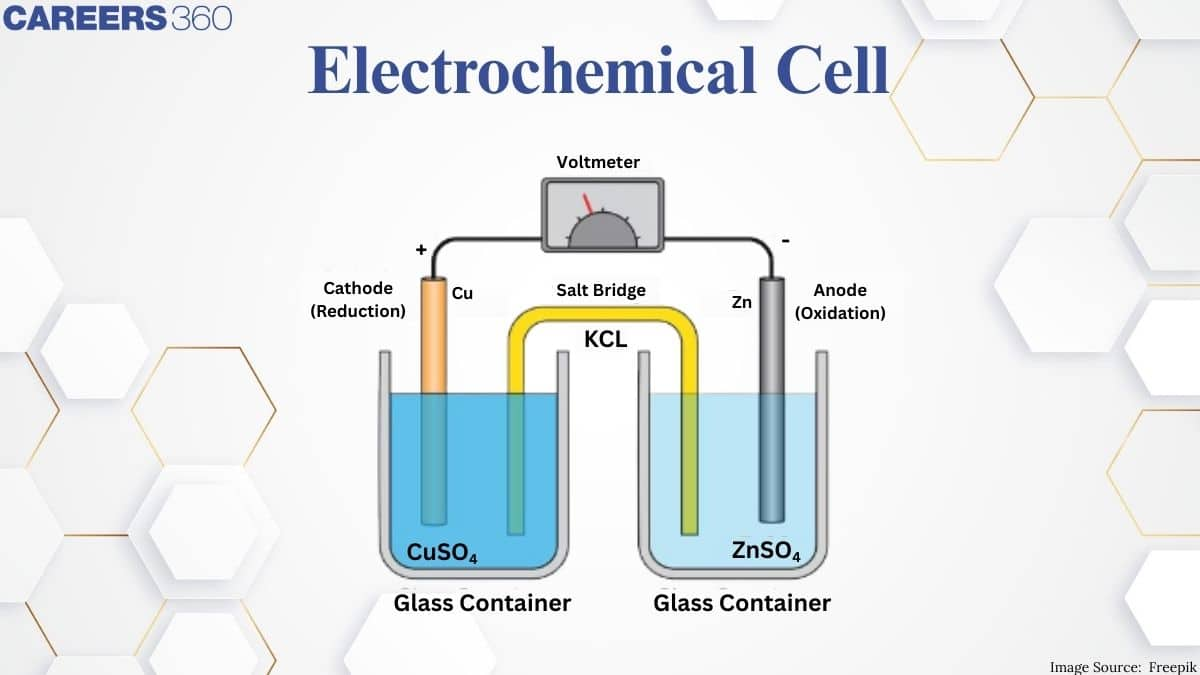
When the anode and cathode are connected by wire, electrons flow from the anode to the cathode. Normal galvanic cell A typical arrangement of half cells connected to form a galvanic cell. Using genetic engineering, let's assemble a cell. One beaker contains 0.15 M Cd (NO3) 2 and Cd metal electrodes. The other beaker even contains 0.20 M AgNO3 and usually the Ag metal electrode. In response, the silver ion is reduced by receiving an electron, and the solid Ag is the cathode. Cadmium is connected by loss of electrons, and a strong Cd is anode.
Electrochemical Cell Diagram
Also read :
Half-Cells and Cell Strength
Electrochemical cells are made up of two and a half cells, each containing an electrode embedded in the electrolyte. The same electrolyte can also be used for both the half cells.
These half cells are connected by a salt bridge that provides an ionic communication platform between them without allowing them to combine. An example of a salt bridge is filter paper coated with potassium nitrate or sodium chloride solution.
One half part of an electrochemical cell loses electrons due to oxidation and the other gains electrons in the degradation process. It can be known that the equilibrium reaction occurs in both half cells, and when equilibrium is reached, the network voltage becomes 0 and the cell stops producing electricity.
The tendency of the electrode to contact the electrolyte for the loss or acquisition of electrons is explained by its electrode strength. The values of this energy can be used to predict the overall strength of a cell. Typically, the electrode strength is measured with the help of a standard hydrogen electrode as a reference electrode (known electrode).
Lower and Second Cells
The primary cells actually use and dislodge galvanic cells. The electrochemical reactions which take place in these cells are irreversible. Therefore, reactants are used for the production of electrical energy and the cell stops producing electricity when the reactants are completely depleted. Second cells (also known as rechargeable batteries) are electrical cells in which the cell has a flexible response, i.e. the cell can function as a Galvanic cell and an Electrolytic cell. Most primary batteries (many cells connected to a series, similarities, or combinations of the two) are considered to be harmful and environmentally damaging devices. This is because they require about 50 percent of the energy they contain in their production process. They also contain a lot of toxic metals and are considered hazardous waste.
Types of Electric Cells
There are two main types of electrochemical cells that are:
1. Galvanic cells (that are also called as Voltaic cells)
2. Electrical cells
The three main components of electrolytic cells are:
Cathode (negatively charged by electrolytic cells)
Anode (charged well with electrolytic cells)
Electrolyte
The electrolyte provides the electron exchange between the cathode and the anode. The most widely used electrolytes in electrolytic cells include water (containing dissolved ions) and molten sodium chloride.
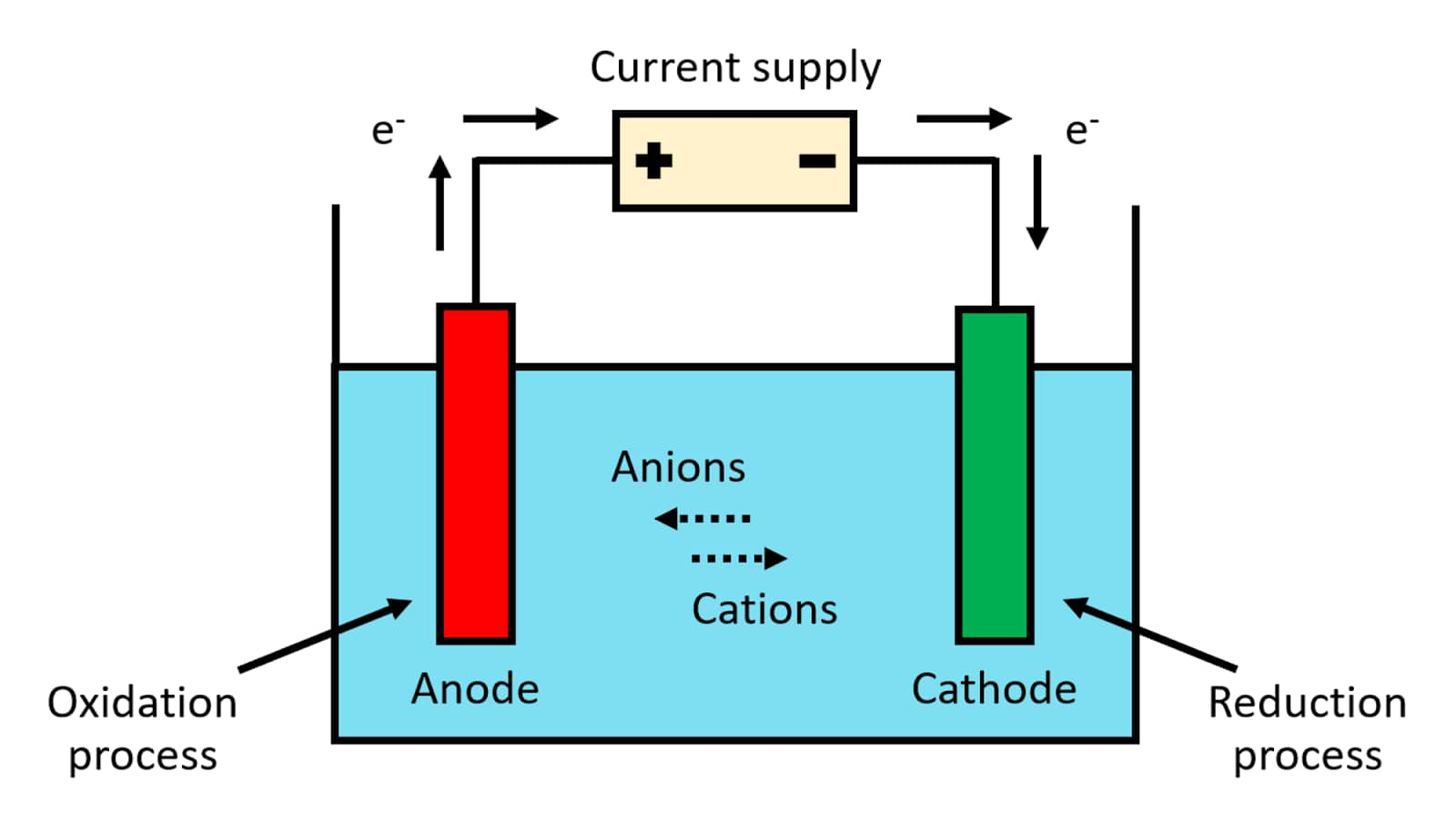
Electrolytic Cell Function
Melted sodium chloride (NaCl) can be subjected to electrolysis. Here, two inert electrodes are placed in molten sodium chloride (containing Na + cations separated from Cl- anions). When electrical energy is transferred to a region, the cathode becomes rich in electrons and has a negative charge. The well-charged sodium cation is now attracted to the badly charged cathode. This causes the formation of metallic sodium in the cathode. At the same time, chlorine atoms are attracted to a well-charged cathode. This causes the formation of chlorine gas (Cl2) in the anode (corresponding to the release of two electrons, terminating the circuit). The corresponding chemical estimates and overall cell response are given below.
Cathode reaction: $\left[\mathrm{Na}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Na}\right] \times 2$
Anode reaction: $2 \mathrm{Cl}^{-} \rightarrow \mathrm{Cl}_2+2 \mathrm{e}^{-}$
Cell Response: $2 \mathrm{NaCl} \rightarrow 2 \mathrm{Na}+\mathrm{Cl}_2$
Therefore, the molten sodium chloride can be subjected to electrolysis in an electrolytic cell to produce metallic sodium and chlorine gas as products.
|
Related Topics link |
Electrolytic Cell Applications
The primary use of electrolytic cells is to produce oxygen gas and hydrogen gas in water. They are used to extract aluminum from bauxite. Another notable use of electrolytic cells is electroplating, which is the process of forming a thin protective layer of a particular metal on the surface of another metal. Electrorefining of most non-ferrous metals is performed with the help of electrolytic cells. Such electrical cells are also used in electrowinning processes. It can be noted that the industrial production of very pure copper, pure zinc, and high-grade aluminum is regularly performed by electrolytic cells.
1. Electroplating
-
Coating a metal object with a thin layer of another metal
-
Improves corrosion resistance, appearance, and durability
-
Examples:
-
Chromium plating on car parts
-
Gold plating on jewellery
-
2. Electrorefining (Purification of Metals)
- Used to obtain high-purity metals
- Impure metal acts as anode; pure metal is deposited at cathode
- Examples: Refining of copper, silver, gold
3. Electrometallurgy (Extraction of Metals)
-
Extraction of highly reactive metals from molten salts
-
Examples:
- Aluminium from Al₂O₃ (Hall–Héroult process)
- Sodium from molten NaCl (Downs process)
4. Manufacture of Chemical Compounds
- Large-scale production of important chemicals
- Examples: NaOH, Cl₂, and H₂ by electrolysis of brine and H₂ and O₂ by electrolysis of water
5. Anodization
- Electrolytic oxidation of aluminium
- Forms a protective oxide layer on the surface
- Used in aircraft parts, utensils, and window frames
6. Electroforming
- Making precise metal objects by electrodeposition
- Used in printing plates, moulds, and micro-components
Also read -
Some Solved Examples
Question 1: Which of the following statement is not correct about an inert electrode in a cell?
1) It does not participate in the cell reaction.
2) It provides surface either for oxidation or for the reduction reaction.
3) It provides a surface for conduction of electrons.
4) (correct) It provides a surface for a redox reaction.
Solution:
The answer is option (4). Inert electrodes act only as a source or sink for electrons. They do not undergo redox reactions and merely provide the surface for the reaction.
Hence, the answer is option (4).
Question 2: What will happen during the electrolysis of an aqueous solution of by using platinum electrodes?
(i) Copper will deposit at the cathode.
(ii) Copper will deposit at the anode.
(iii) Oxygen will be released at the anode.
(iv) Copper will dissolve at the anode.
1) (i) and (ii)
2) (iii) and (iv)
3) (ii) and (iii)
4) (correct) (i) and (iii)
Solution:
The answer is option ( i, iii ).
$\begin{aligned}
& \mathrm{CuSO}_4 \rightleftharpoons \mathrm{Cu}^{2+}+\mathrm{SO}_4^{2-} \\
& \mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}^{+}+\mathrm{OH}^{-}
\end{aligned}$
At cathode: $\mathrm{Cu}^{2+}+2 e^{-} \rightarrow \mathrm{Cu} ; E_{\text {cell }}^0=0.34 \mathrm{~V}$
$H^{+}+e^{-} \rightarrow \frac{1}{2} H_2 ; E_{\text {cell }}^0=0.00 \mathrm{~V}$
At the cathode, the reaction with higher $E^0$ is preferred
At anode: $2 \mathrm{SO}_4^{2-}-2 e^{-} \rightarrow S_2 O_8^{2-} ; E_{\text {cell }}^0=1.96 \mathrm{~V}$
$2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{O}_2+4 \mathrm{H}^{+}+4 e^{-} ; E_{\text {cell }}^0=1.23 \mathrm{~V}$
At the anode, the reaction with lower $E^o$ is preferred.
Hence, the answer is option (4).
Question 3: In an electrochemical cell formed by a Zn and a Cu rod:-
1) Zn behave as a cathode, and Cu behaves as an anode
2) (correct) Zn behave as anode, and Cu behaves as cathode
3) Either Zn or Cu can behave as an anode or cathode
4) None of these
Solution:
As we learned
Electrochemistry -
It is the study of the production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations.
For $\left(Z n_e\right)$ value of SOP is more and for $C u$ value of SRP is more. So $Z n$ will behave as anode and $C u$ will behave as cathode.
Hence, the answer is option (2).
Question 4: Which of the following can be classified as a chemical cell?
1) Electrolytic cell
2) Galvanic cell
3) Daniel Cell
4) (correct) 2 & 3
Solution:
Chemical Cells - The cells in which electrical energy is produced from the energy change accompanying a chemical reaction or a physical process are known as chemical cells.
The Electrolytic cell is a device in which electrolysis is carried out by using electricity or in which the conversion of electrical energy into chemical energy is done.
The Galvanic cell is a device in which the redox reactions lead to the conversion of chemical energy into electrical energy.
A Daniell cell is a galvanic cell that converts chemical energy into electrical energy by using the spontaneous redox reaction between zinc and cupric ions.
Hence, the answer is option (4).
Practice more questions with the link below
Frequently Asked Questions (FAQs)
The cellular response of electrolytic cells does not occur spontaneously and the reaction of Galvanic cells is spontaneous. Galvanic cells produce electrical energy from chemical reactions and electrolytic cells produce spontaneous redox reactions from electrical input.
The three main components which are available in electrolytic cells are the cathode, anode, and electrolyte. In electrolytic cells (as is the case with most electrochemical cells), oxidation occurs at the anode and decomposition occurs at the cathode.
In electrolytic cells, the cathode is poorly charged and the anode is well charged. Well-charged ions flow to the cathode and poorly charged ions flow to the anode.
Electrolytic cells can be used to produce oxygen gas and hydrogen gas in water by moving it into electrolysis. These devices can also be used to obtain chlorine gas and metallic sodium from strong solutions of sodium chloride (common salt). Another important use of electrolytic cells is electroplating.
When external electrical energy flows into the cathode of an electrolytic cell, the negatively charge attracts the separated ions present in the electrolyte. This results in the insertion of well-charged ions into the cathode. At the same time, negatively charged ions flow into the anode, charged well.
