Metals, Non-metals and Metalloids
In chemistry, elements are categorized into metals, non-metals, and metalloids, based on their observable physical and chemical features. The majority of metals occupy the left and central regions of the periodic table and exhibit traits such as metallic luster, malleability, electrical and thermal conductivity, and the tendency to lose electrons. Non-metals, found toward the right side of the table, lack these metallic characteristics and often form negative ions during reactions. Metalloids lie along the diagonal boundary that separates metals from non-metals, displaying a mix of both sets of properties.
This Story also Contains
- From Shine to Snap: Exploring Metals, Non‑metals & Metalloids
- Metals
- Non-metals
- Metalloids
- Some Solved Examples
- Conclusion
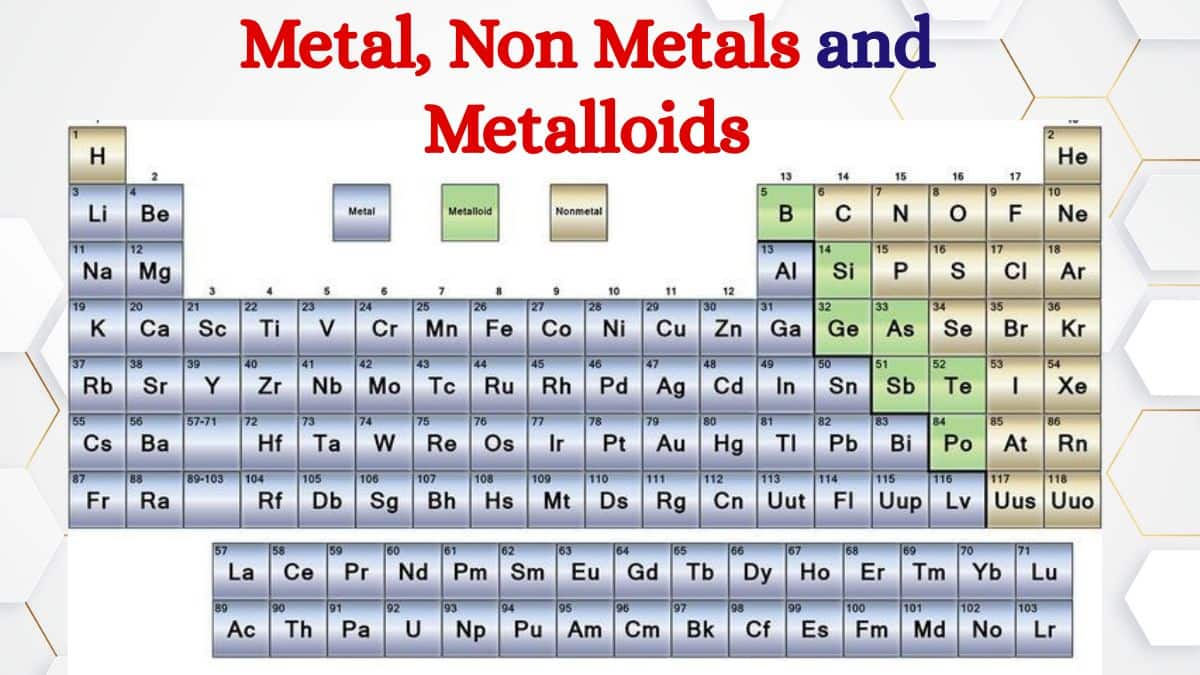
This classification—part of the broader unit on the classification of elements—is a major topic in Class XI Chemistry. It not only helps students in board-level exams but also plays a vital role in entrance tests like JEE Main, NEET, BITSAT, WBJEE, SRMJEE, BCECE, and others. Notably, this topic featured in JEE 2020 over the past decade.
Also read -
From Shine to Snap: Exploring Metals, Non‑metals & Metalloids
Elements are broadly categorized into metals, non‑metals, and metalloids based on their shared chemical and physical traits.
Metals account for the majority of known elements (roughly 75–80%) and are concentrated on the left and center of the Periodic Table
At standard conditions, metals are typically solid. Notable exceptions include mercury, which is liquid at room temperature, and low-melting metals like gallium and cesium, which liquefy just above room temperature (~29–30 °C, or ~302–303 K) .
Most metals have high melting and boiling points because of strong metallic bonding, where positively charged ions are held tightly within a “sea” of mobile electrons.
They excel at conducting heat and electricity, a result of the free electrons that easily transfer energy through the metallic structure.
Metals are also malleable and ductile; this means they can be shaped into sheets or drawn into wires without breaking, thanks to the ability of atomic layers to slide over one another while maintaining the metallic bond.
Metals
Most elements in the periodic table are classified as metals, found to the left of the familiar zig-zag (or stair-step) line that visually separates metallic from nonmetallic regions. Along this line—and sometimes just to its left—are the metalloids (also known as semimetals), such as boron, silicon, germanium, arsenic, antimony, tellurium, and polonium. Their location signals that they blend the traits of both metals and nonmetals, occupying an intermediate position in terms of properties.

Non-metals
They are placed at the top right side of the Periodic Table. In a period, the properties of elements change from metallic on the left to non-metallic on the right. Non-metals are usually solids or gases at room temperature with low melting and boiling points (boron and carbon are exceptions). They are poor conductors of heat and electricity. Most non-metallic solids are brittle and are neither malleable nor ductile. The elements become more metallic as we move from top to bottom in a group and the non-metallic character increases as we move from left to right in a period.

Metalloids
Certain elements exhibit properties that are intermediate between metals and nonmetals; these are known as metalloids. Typically, metalloids are found along a diagonal line on the periodic table, which separates metals from nonmetals. This line extends from boron (B) at the top left to astatine (At) at the bottom right. The eight most commonly recognized metalloids are:
Boron (B)
Silicon (Si)
Germanium (Ge)
Arsenic (As)
Antimony (Sb)
Tellurium (Te)
Polonium (Po)
Astatine (At)
These elements possess characteristics that are a blend of both metals and nonmetals. For instance, they often have a metallic appearance and are solid at room temperature, but they are more brittle than metals and are less efficient conductors of electricity. Chemically, metalloids can behave like nonmetals, forming covalent bonds and displaying intermediate electronegativity values. Due to these unique properties, metalloids are valuable in various applications, including semiconductors, solar cells, and as alloying agents in metallurgy.

Also Read:
Recommended topic video on(Metals, non-metals and Metalloids):
Some Solved Examples
Example 1: Generally, metals react with acids to give salt and hydrogen gas. Which of the following acids does not give hydrogen gas on reacting with metals (except Mn and Mg)?
1) H2SO4
2) HCl
3) HNO3
4) All of these
Solution: Classification of Elements as Metals, Non-metals and Metalloids -
The elements can be divided into three categories, i.e., metals, non-metals, and metalloids. Metals comprise more than 78% of all known elements and appear on the left side of the Periodic Table. Metals are usually solids at room temperature except for mercury, which is liquid at room temperature, and even gallium and caesium also have very low melting points (303K and 302K., respectively). Metals usually have high melting and boiling points. They are good conductors of heat and electricity. They are malleable and ductile.
Nitric acid(HNO3) is a strong oxidising agent. The Hydrogen gas produced during its reaction with metal gets oxidised to H2O, hence no hydrogen gas is produced.
Hence, the answer is the option (3).
Example 2: The lightest metal in the periodic table is :
1) Ca
2) Hg
3) Na
4) Li
Solution: Metals -
– Form metallic bonds.
– 78% of all known elements.
– good conductor of heat & electricity.
– Malleable and ductile.
Li is the lightest metal in the periodic table.
Hence, the answer is the option (4).
Example 3: Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): Metallic character decreases and non-metallic character increases on moving from left to right in a period.
Reason (R): It is due to an increase in ionisation enthalpy and a decrease in electron gain enthalpy, when one moves from left to right in a period.
In the light of the above statements, choose the most appropriate answer from the options given below :
1) (A) is false but (R) is true.
2) Both (A) and (R) are correct and (R) is the correct explanation of (A).
3) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
4) (A) is true but (R) is false.
Solution: On moving from left to right in a period, the metallic character decreases while the non-metallic character increases. This is due to an increase in Ionisation enthalpy and an increase in the magnitude of electron gain enthalpy.
Thus, Assertion (A) is true while Reason (R) is false
Hence, the answer is the option (4).
Example 4: The lustre of a metal is due to
1) It's high polishing
2) Its high-density
3) Chemical inertness
4) Presence of free electrons
Solution: As we learned in, Metallic solids -
Metallic bond, i.e., the attraction between the positively charged metal ion and mobile electron.
Ex. iron, copper, zinc, aluminium, sodium
The metallic lustre of a metal is due to the presence of free electrons.
Hence, the answer is the option (4).
Example 5: Which is the only metal that exists in liquid form at room temperature?
1) Br2
2) Ag
3) W
4) Hg
Solution: Hg as mercury exists as a liquid at room temperature.
Hence, the answer is an option (4).
Example 6: Which one of the elements is a non-metal?
1) C
2) Al
3) Na
4)Cr
Solution: Non- Metal -
– Located at the right of the Periodic Table
– Poor conductor of heat & electricity
– They are usually solids or gases at room temperature.
C is a non-metal
Hence, the answer is the option (1).
Example 7: Given below are two statements:
Statement I: Both metals and non-metals exist in p and d-block elements.
Statement II: Non-metals have higher ionization enthalpy and higher electronegativity than the metals.
In the light of the above statements, choose the correct answer from the options given below:
1) Both statement I and statement II are false
2) Both statement I and statement II are true
3) Statement I is false but statement II is true
4) Statement I is true but statement II is false
Solution:
Statement I: p-block has Metal as well non metals. while d-block has only metal. hence Ist is incorrect. Statement II : Non-Metal has high I.E. & E.N.
F→ highest E.N.
He→ Highest I.E.
Hence, the answer is the option (3).
Example 8: Which of the following elements is considered a metalloid?
1) Sc
2) Pb
3) Bi
4) Te
Solution: Fact Based.
Sc,Pb and Bi are metals while Te is a metalloid.
Hence, the answer is the option (4).
Example 9: Which of the elements are metalloids?
1) B
2) Si
3) Sb
4) All of these
Solution: Metalloids or Semi-metals -
They show the properties of both metals and non-metals. Their change from metallic to non-metallic character is non-abrupt.
B, Si, and Sb all are metalloids.
Hence, the answer is the option (4).
Practice more Questions from the link given below:
Conclusion
Therefore, the division of elements in terms of metals, non-metals, and metalloids gives a primary understanding of the various parts of these aspects of chemistry. Some metals have shining surfaces, electrical conductivity and ductility, and they are used in manufacturing industries, structuring and electrical industries as well. Some of the main applications of non-metals which are described as brittle and poor conductors of heat and electricity include roles in biosystems, for environmental applications, and as the constituent of different compounds and materials. Metalloids; chemical elements displaying characteristics of both metals and non-metals find critical utilization in the technology of semiconductors which culminated in the state-of-the-art technological inventions in electronics and computers.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
Frequently Asked Questions (FAQs)
Group 14 is unique in that it contains this full spectrum—metals, metalloids, and a nonmetal—all in one family.
The seven widely recognized metalloids are:
Boron (B)
Silicon (Si)
Germanium (Ge)
Arsenic (As)
Antimony (Sb)
Tellurium (Te)
Polonium (Po)
These elements lie along the “stair-step” between metals and nonmetals on the periodic table and exhibit a mix of metallic and nonmetallic properties—like semiconducting behavior, brittleness, and intermediate electronegativity .
Ductility signifies a metal's capacity to be drawn into wires. It also relies on the flexible atomic lattice and mobile electrons that allow deformation without fracture.
The “sea of electrons” in metals allows electrons to move freely throughout the structure, enabling rapid transfer of both heat and electric charge .
Metalloids play a crucial role in modern technology, particularly in the electronics industry. Their applications include:
Semiconductors: Silicon and germanium are foundational in the manufacture of computer chips and transistors.
Solar cells: Silicon is extensively used in photovoltaic cells to harness solar energy.
Alloys: Metalloids like antimony and arsenic are added to metals to enhance properties such as strength and resistance to corrosion.
Flame retardants: Compounds containing metalloids are used to reduce flammability in materials.
Mercury is the only metal which liquid at room temperature.
Non-metals are commonly solid or gaseous at room temperature and have low melting and boiling points.
Metalloids are commonly used in semiconductors, alloys and biological agents.
Metalloids are commonly used in semiconductors, alloys and biological agents.
Palladium is amongst the four most expensive metals besides Gold, Silver and Rhodium.
