Ellingham Diagram
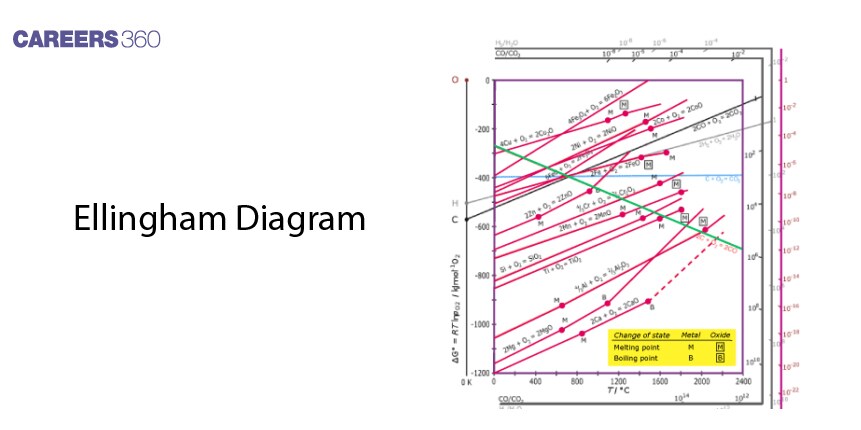
The Ellingham diagram is an important aid that helps in the prediction of the temperatures needed when reducing metal oxides to pure metals, and, in so doing, assists metallurgists when deciding on the most effective way to extract it. Smelting is one of the earliest processes in history that consists of heating ore to high temperatures to recover metals by reduction with such agents as carbon. Meanwhile, in the Thermite Process, tremendous heat is liberated through possibly exothermic oxidation-reduction reactions between highly reactive metals such as aluminum with metal oxides.
This Story also Contains
- Ellingham Diagram
- Reduction of Oxides to Metal: Smelting
- Reduction by a More Electropositive Metal: Thermite Process
- Everyday Connection, Importance, and Applications
- Some Solved Examples
- Summary
Ellingham Diagram
Consequently, the Ellingham diagram is of prime importance for metallurgists to understand the thermodynamic functionality of reduction of metal oxides. Geometrically, it is a plot of ΔG° for oxide formation against temperature. The lower the line is on the diagram, the more easily reducible the oxide is to its metal. Therefore, every plot is a straight line, which slopes upwards, showing the change in Gibbs free energy against temperature.
Function and Interpretation
The Ellingham diagram is a graph between temperature and the change in Gibbs free energy on the y-axis and x-axis, respectively. All the lines of various metal oxides indicate their stability with temperature. A very important point of the diagram would be where a line crosses the axis ΔG°=0, which gives the temperature above which an oxide will decompose spontaneously.
Limitations
While the Ellingham Diagram depicts the thermodynamic feasibility, it does not reveal information about the reaction kinetics. Furthermore, it is inferred assuming equilibrium conditions, which may significantly differ from reality, especially for heterogeneous processes that involve solids.
Reduction of Oxides to Metal: Smelting
Smelting Overview
Smelting is an old, now-replaced process for extracting metals from their ores through heating and melting. It is the reduction of metal oxides using a reducing agent like carbon or carbon monoxide.
Smelting Process
1. Pre-treatment of Ore: Ores are crushed and ground formally to liberate the metal-containing minerals.
2. Reduction: Pre-treated or prepared ore is charged into the furnace and heated with a reducing agent, thus reducing the metal oxide to molten metal.
3. Refinement: The molten metal is further refined to remove any impurities.
Example: Smelting in Tin
In tin, concentrated cassiterite ores of the form SnO2 are mixed with anthracite powder and powdered limestone
to give the following mixture. The heating of this mixture, usually in a reverberatory furnace, will reduce the ore to tin and the impurities combine with silica to be removed as calcium silicate (slag). The thus obtained tin is called black tin and is only 99.5% pure.[SnO2 + O2 ]
Reduction by a More Electropositive Metal: Thermite Process
Thermite Process Overview
The thermite process for reducing metal oxides involves making use of more electropositive metals like aluminum. This is an exothermic reaction so much heat is released and therefore has applications where high temperatures are sought.
Thermite Reaction
A typical thermite reaction involves aluminum powder and iron(III) oxide:
Initially, this produces molten iron and aluminum oxide, with temperatures over 2500°C.
Applications
The Thermite Process is utilized in welding rail tracks, metal cutting, and fireworks because it produces high-intensity heat and light.
Everyday Connection, Importance, and Applications
Industrial Uses
1. Steel Production: Smelting and refining are an essential part of the manufacture of fine-quality steel that gets used in building and construction, automobiles, etc.
2. Electronics: Purifying metals like silicon and copper assures the manufacture of effective electronic components.
3. Welding and Repair: The Thermite Process is invaluable in the maintenance of railway tracks and other applications in welding using high temperatures.
Academic Significance
Very useful concepts in chemistry and material science, give some valuable insights into chemical reactions and thermodynamics, as well as into engineering and industry-based applications.
Recommended topic video on(Ellingham Diagram)
Some Solved Examples
Example 1
Question: What is the primary information represented by the Ellingham Diagram?
1. Change of \(\Delta H\) with temperature.
2. Change of \(\Delta G\) with pressure.
3. Change of (\(\Delta G - T\Delta S\)) with temperature.
4. Change of \(\Delta G\) with temperature.
Solution: The Ellingham Diagram represents the change in Gibbs free energy (\(\Delta G\)) with temperature for the formation of metal oxides. Hence, the correct answer is option 4.
Example 2
Question: With respect to an ore, what feasibility does the Ellingham Diagram help to predict?
1. Vapor phase refining.
2. Thermal reduction.
3. Zone refining.
4. Electrolysis.
Solution: The Ellingham Diagram helps to predict the feasibility of the thermal reduction of an ore. Hence, the correct answer is option 2.
Example 3
Question: Which metal cannot be extracted by the smelting process?
1. Zn
2. Cr
3. Pb
4. Fe
Solution: Chromium (Cr) cannot be extracted by the smelting process as it is more reactive and cannot be reduced by carbon or coke. Hence, the correct answer is option 2.
Example 4
Question: What is the purpose of smelting an ore?
1. To oxidize it.
2. To reduce it.
3. To separate volatile impurities.
4. To obtain an alloy.
Solution: The purpose of smelting an ore is to reduce the metal oxide to its pure metal form. Hence, the correct answer is option 2.
Example 5
Question: Which process is used for joining broken pieces of heavy iron objects like girders and railway tracks?
1. Hall-Heroult process.
2. Calcination process.
3. Thermite Process.
4. Mond process.
Solution: The Thermite Process is used for joining broken pieces of heavy iron objects, such as girders and railway tracks, through a highly exothermic reaction. Hence, the correct answer is option 3.
Summary
Ellingham Diagram, Smelting, and the Thermite Process are very cardinal subjects in the extraction of metals and their purification. The Ellingham Diagram predicts the feasibility of reduction, whereas smelting and the Thermite Process are standard techniques to perform the extraction and purification. These have wide applications in industries and thus are critical in order to land a job in both chemistry and metallurgy, as well as to be counted amongst the professionals.
Frequently Asked Questions (FAQs)
The output from the smelting furnace has applications in structural works, electronics, and for many manufacturing industries. The products of the thermite process are very useful in welding, cutting metals at high temperatures, and pyrotechnics-related things.
The Ellingham diagram provides a graphical plot in which information about the free energy change of metal oxide formation, and thus, can be defined the graph as a function of temperature with the temperatures at which metal oxides are capable of reduction to pure metal.
Smelting refers to the process of heating and melting alongside ores performed onto a reducing agent for obtaining pure metal. The process includes the preparation of the ores, reduction within the furnace, and then the purification of the molten metal.
It is the exothermic reaction in which one metal is more electropositive, including aluminum, used to remove the oxide of a less electropositive metal such that pure metal leaves behind. The process finds valuable applications that use very high temperatures like in welding, metal cutting, and so on.
An Ellingham Diagram is used by metallurgists to choose the best-suited reduction techniques, due to its prediction ability to check the feasibility of reactions, using a temperature and changes in Gibbs free energy.
