Refining Process Against Impurities
Metal refining is the process of purification of metal obtained from its ore and gained with impurities, aimed at increasing the purity for it to be suitable for various uses. Crucial refining processes make sure that metals regulate the required standards of different industries, technology, and items of consumer use. The method involves the removal of impurities, which cause variation in properties plus the performances of metals.
This Story also Contains
- Different Types of Refining Processes
- Relevance and Applications
- Some Solved Examples
- Summary:
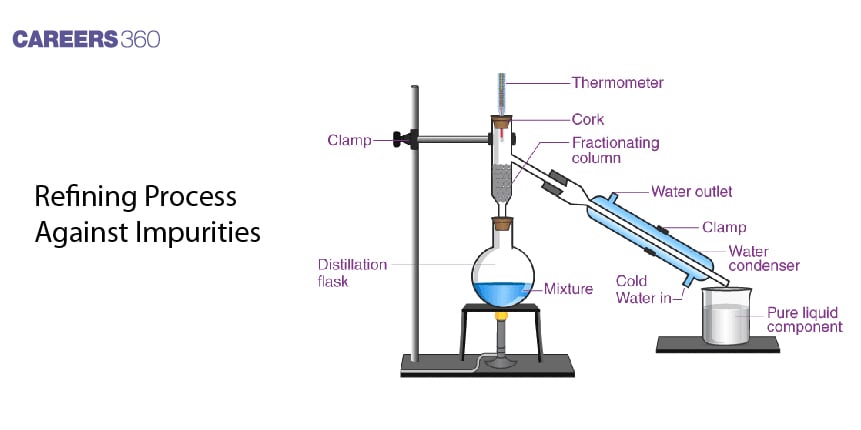
Procedures involved in refining separate the wanted metal, without impurities and undesired elements. Ranging from physical to chemical methods, each is applied to metals with special properties that enable such a method of refinement to be employed. The choice of process thus depends on the melting point, boiling point, reactivity, and the nature of its impurities.
Different Types of Refining Processes
Liquation
Liquation is a process used for purifying metals with low melting points like tin. Here, the impure metal is heated on an inclined plane. While the metal melts, it flows down the plane and leaves behind the high melting impurities. This method is good at separating metals from less fusible impurities. It is mainly used in the refining of tin.
Distillation Method
Especially, it serves metals with a low boiling point like zinc and mercury. The impure metal is heated till vaporization takes place. On condensation, the metal vapor thus obtained yields the pure metal as distillate. Such a method separates metal from non-volatile impurities.
Electrolytic Refining
Electrolytic refining is a process of purification of metals using an electric current. In the process, the impure metal will act as the anode while the strip of pure metal acts as the cathode. The electrodes will be immersed in an electrolytic bath containing a soluble salt of the same metal. When electricity flows, the impure metal will dissolve from the anode into the solution and deposit as pure metal on the cathode. For instance, copper is purified using this process where at a current, impure copper acts as the anode while pure copper acts as the cathode in an acidified copper sulfate solution. The impurities settle as anode mud which may contain valuable metals such as silver, gold, and platinum.
In this method, the impure metal is made to act as anode. A strip of the same metal in pure form is used as a cathode. They are put in a suitable electrolytic bath containing soluble salt of the same metal. The more basic metal remains in the solution and the less basic ones go to the anode mud. This process is also explained using the concept of electrode potential, over potential, and Gibbs energy which you have seen in previous sections. The reactions are:
Anode:$\quad \mathrm{M} \rightarrow \mathrm{M}^{\mathrm{n}+}+\mathrm{ne}^{-}$ M→Mn++ne−
Cathode: $\quad \mathrm{M}^{\mathrm{n}+}+\mathrm{ne}^{-} \rightarrow \mathrm{M}$ Mn++ne−→M
Copper is refined using an electrolytic method. Anodes are of impure copper and pure copper strips are taken as cathode. The electrolyte is acidified solution of copper sulphate and the net result of electrolysis is the transfer of copper in pure form from the anode to the cathode:
Anode: $\quad \mathrm{Cu} \rightarrow \mathrm{Cu}^{2+}+2 \mathrm{e}^{-}$
Cu→Cu2Cathode: $\mathrm{Cu}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}$ Cu2++2e−→Cu
Impurities from the blister copper deposit as anode mud which contains antimony, selenium, tellurium, silver, gold, and platinum; recovery of these elements may meet the cost of refining. Zinc may also be refined this way.
Zone Refining Process
Zone refining is a process for the production of ultra-pure metals. A movable heater is applied to a rod of impure metal and melts it locally. As the heater travels along the rod, the pure metal crystallizes out, but the impurities are carried along with the melted zone. This process, when repeated a number of times, gives metals of high purity, and it is particularly useful for the purification of semiconductors or other materials where very high purity is required.
A movable heater is fitted around a rod of impure metal. The heater is slowly moved across the rod. The metal melts at the point of heating and as the heater moves on from one end of the rod to the other end, the pure metal crystallizes while the impurities pass on the adjacent melted zone.

Vapor Phase Refining
It is a process involving the conversion of the metal into a volatile compound and its subsequent decomposition to get back the pure metal. The process mandated the fact that the metal should form a volatile compound with some readily available reagent and that the formed compound be decomposed easily.
In this method, the metal is converted into its volatile compound and collected elsewhere.
It is then decomposed to give pure metal. So, the two requirements are:
(i) the metal should form a volatile compound with an available reagent,
(ii) the volatile compound should be easily decomposable so that the recovery is easy.
The following examples will illustrate this technique.
Mond Process for Refining Nickel: In this process, nickel is heated in a stream of carbon monoxide forming a volatile complex, nickel tetracarbonyl:
Ni+4CO→330−350 KNi(CO)4
The carbonyl is subjected to higher temperature so that it is decomposed giving the pure metal:
Ni(CO)4→450−470 KNi+4CO
Van Arkel's Method for Refining Zirconium or Titanium: This method is very useful for removing all the oxygen and nitrogen present in the form of impurities in certain metals like Zr and Ti. The crude metal is heated in an evacuated vessel with iodine. The metal iodide being more covalent, volatilises:
Zr+2I2→ZrI4
The metal iodide is decomposed on a tungsten filament, and electrically heated to about 1800K. The pure metal is thus deposited on the filament.
Relevance and Applications
Real-Life Applications
Several purification procedures are involved in various applications that touch everyday life. One such excellent example could be the ultrapure silicon used in computers and smartphones, which comes out through zone refinement. This guarantees very minimal impurities within the silicon, extremely necessary for its semiconducting actions. Similarly, copper is used in electrical wiring that undergoes electrolytic refinement to attain the required conductivity and durability.
Industrial Significance
These refining processes are also responsible for the quality standards of metals used in manufacturing processes in the industrial sector. For example, aerospace industries use high-purity titanium obtained using the Van Arkel method to derive lightweight, yet strong components. Refined metals like aluminum and steel are playing a wide role in the application of the automotive industry while building resistant, yet fuel-efficient vehicles.
Academic Importance
The various types of refining processes in the domain of materials science and metallurgy are quite important to know. They get studied not only for developing new techniques to improve the achievable levels of purity but also for the areas that bring improvement in known techniques for better efficiency. Students and researchers who partake in this field bring into consideration associated thermodynamics and kinetics with the processes of refining to achieve innovation and optimization of metal extraction and purification techniques.
Recommended topic video on (Refining Process Against Impurities )
Some Solved Examples
Example 1
Question: Which method is suitable for the refining of Bi?
1) Liquation (correct)
2) Zone refining
3) Electrolysis
4) All
Solution: Liquation is the method most suitable for refining bismuth (Bi) due to its low melting point. This process exploits the difference in melting points between bismuth and its impurities. By heating the impure metal just above the bismuth's melting point, the metal melts and flows away, leaving the higher-melting impurities behind. Hence, the correct answer is option 1.
Example 2
Question: Refining using the liquation method is most suitable for metals with:
1) Low melting point (correct)
2) High boiling point
3) High electrical conductivity
4) Less tendency to be soluble in melts than impurities
Solution: The liquation process is based on the principle that metals with a lower melting point than their impurities will melt and separate first. This method is therefore suitable for metals like bismuth, tin, lead, and mercury, which have lower melting points. Hence, the correct answer is option 1.
Example 3
Question: A mixture of Zn and Hg can be separated by:
1) Electrolysis
2) Distillation (correct)
3) Vapour Phase refining
4) Zone refining
Solution: Distillation is suitable for separating a mixture of zinc (Zn) and mercury (Hg) due to their differing boiling points. Mercury, with a lower boiling point of approximately 356.7°C, vaporizes first and can be collected separately from zinc, which has a boiling point of approximately 907°C. Hence, the correct answer is option 2.
Example 4
Question: Which metals are refined by the distillation process?
1) Ni, Al
2) Na, Mg
3) Fe, Al
4) Zn, Cd (correct)
Solution: The distillation process is very useful for refining low-boiling metals such as zinc and cadmium. These metals vaporize at relatively low temperatures, allowing them to be separated from impurities that remain solid. Hence, the correct answer is option 4.
Example 5
Question: Aluminium is extracted by the electrolysis of:
1) Bauxite
2) Alumina
3) Alumina mixed with molten cryolite (correct)
4) Molten cryolite
Solution: Aluminium is extracted from alumina (Al2O3) using the Hall-Héroult process, where alumina is dissolved in molten cryolite (Na3AlF6). This mixture is then subjected to electrolysis, which significantly lowers the melting point and improves the conductivity of alumina. Hence, the correct answer is option 3.
Summary:
In addition to the paramount process in purity and quality assurance for metals being refined, the various methods discussed, such as liquation, distillation, electrolytic refining, zone refining, and vapor phase refining, exhibit unique advantages and fields of application. Charges are taken out together with enhancements on the properties of metals to suit them in their technology, industry, and product applications used in daily life. Knowing these methods of purification makes one appreciate the process taken by the extracted raw ore to become a metal this incredibly high in purity, that makes our modern world work.
Frequently Asked Questions (FAQs)
Zone refining has special importance in semiconductors due to its ability to produce ultra-pure silicon with less than one part per million impurities. High-purity silicon is demanded due to the exigencies of the performance and reliability of electronic devices.
ZrI4→Zr+2I2
Another process of purification for metals like zirconium and titanium is the van Arkel process. It involves heating the metal with iodine to form volatile metal iodide. The decomposition takes place on a hot filament, which forms pure metal.
The main purpose of metal refining is the purification of impure metals obtained from ores by means of impurity removal to key purity levels for various industrial, technological, and consumer applications.
Electrolytic refining involves the process whereby an impure metal gets dissolved at the anode by a direct electric current with deposition taking place at the cathode in an electrolytic bath. This separates the impurities, which form anode mud.
Also, it is applied in refining metals with low boiling points, such as zinc and mercury. Impure metal is vaporized and then condensed as pure metal.
