Ion Definition - What is an Ion?, Explanation and FAQs
Ion definition
In this article we will discuss what is an ion, what is actual ion meaning, what are the examples of ion, how we define ion, what is ion in chemistry, what are the types of ion, then we will discuss what are the positively charged ions called, what are the negatively charged ions called and how ions are formed and more detailed description about an ion.
What is an ion?
Here firstly we will discuss in general what is an ion. As we all know that in an atom there are two types of charged species electrons and protons and as electrons are more in number and revolve around the nucleus are conventionally considered to be of the negative nature and nucleus is considered of the positive nature and supposed to contain positive species called protons.
As already said that electrons are more in number and therefore outnumber the protons therefore there is some net charge on the atom and that net charge can also be due to some loss or gain of electrons in a molecule from the other atom. When this net charge is present on the atom it is called an ion. now as this is general ion meaning but now we will discuss what is the actual meaning of ion.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
What is an ion in chemistry?
So when we discuss ion meaning in general, we come to know that it is basically an atom or a molecule having a net electric charge on it due to some loss or gain of an electron. this gain or loss can be due to the formation of some required molecule and further in the formation of the compound. As we have discussed ion meaning now keeping that in mind we will quote some basic examples explaining the same.in chemistry we generally write the atomic symbols with their charges like sodium is written as Na+ and chlorine is written as Cl-.
From these examples, we can see that the sodium atom has a positive charge and the chlorine atom has a negative charge so from this we come to know that the positive ions are called a cation and the negative ions are called anions. So, from this, we concluded that there are two types of ions i.e that the positively charged ions are called a cation and the negatively charged ions are called anions.
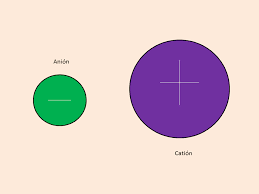
The next question arises that how the ions are formed.
How are ions form?
Talking about the atoms we know that in chemistry we have atoms and atoms are supposed to contain the proton which is positively charged and outside the nucleus contain the negatively charged electrons which balance each other effect and makes the entire atom neutral. In this, if the atom loses one electron then the charge becomes +1 and if the atom gains an electron then it attains -1 charge.
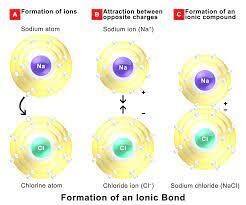
Also read :
Ion ion bonding
As we all know that the chemistry shows different types of bonding between the atoms and such bonding differ depending upon the nature of the atom. as it is well known that opposites attract each other. So this is the main criteria we can say that is applied in the ionic bonding. We define ionic bonding in proper terms as the “ chemical bonding where the electrostatic force of attraction is seen among oppositely charged ions from two different blocks of the periodic table or between the atoms with sharply different electronegativities.
Also check-
