Isotopes of Hydrogen: Definition, Diagram and Examples
Hydrogen has been in existence since interstellar space down to the atoms of every dripping water molecule. Nevertheless, being the most basic and abundant element, its fascination lies in the profound implications it makes in physics and chemistry and probably even extraterrestrial life, in whose search greater interest is evinced today. This chapter serves to introduce the reader to protium, deuterium, and tritium, which are so much less well-known forms of hydrogen than their constituent role in water and an endless array of organic compounds.
This Story also Contains
- About Hydrogen:
- Isotopes of Hydrogen:
- Position and Similarity of Hydrogen in the Periodic Table
- Applications and Relevance of Hydrogen Isotopes
- Some Solved Examples
- Summary
Occurrence of Hydrogen - Isotopes of Hydrogen
Hydrogen is the lightest element and is undoubtedly an extremely important factor in the universe as well as on Earth. Hydrogen makes up about 75% of the elemental mass of the universe, with helium second. On Earth, oxygen forms the bulk by mass, but hydrogen is truly omnipresent coming in water and organic compounds and as a part of thousands of substances.
About Hydrogen:
Although hydrogen stands at the head of the periodic table, it has become customary to remove it in discussions because of the anomalies it causes. This is the lightest atom, having just one proton and one electron in the simplest form. Hydrogen is an elementary atom, and it is thus one of the cornerstones for understanding atomic theory.
In its elemental form, hydrogen is a diatomic molecule H2, commonly called dihydrogen. This molecular shape is stable and very common to several natural and synthetic processes. Hydrogen is a very reactive molecule and forms more compounds than any element of the periodic table; these compounds range from very simple hydrides to highly complex organic compounds.
Isotopes of Hydrogen:
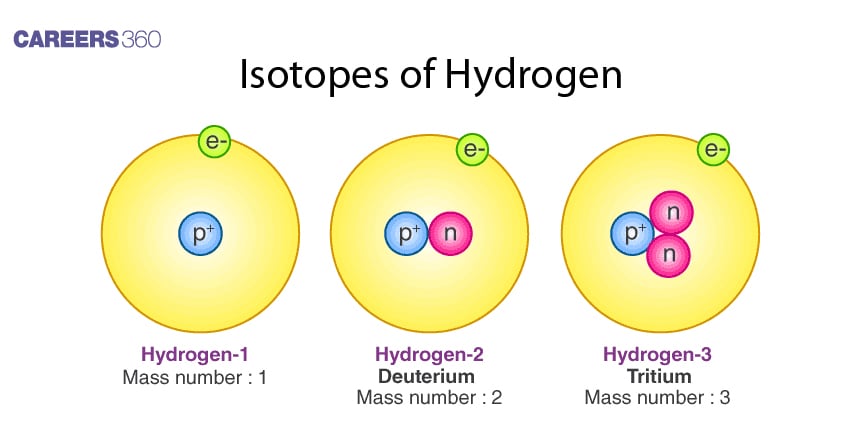
Hydrogen has three isotopes: protium, 1H, deuterium, 2H or D, and finally, tritium, 3H or T. The atoms of these isotopes differ only in their neutron count and share identical electron configurations. Thus, even though they have similar chemical properties, their physical properties are quite disparate due to large mass differences. Tritium is the only radioactive isotope of hydrogen and undergoes beta decay.
Position and Similarity of Hydrogen in the Periodic Table
The position of hydrogen in the periodic table is governed by its dual nature. Its electronic configuration (1s1) resembles both alkali metals with ns1 as well as the halogens ns2 np5. Alkali metals lose one electron to become unipositive ions, similar to hydrogen. The halogens gain one electron to achieve a noble gas configuration like that of helium, which is 1s2.
However, hydrogen differs significantly from alkali metals in that it does not exhibit any typical metallic characteristics, such as conductivity and malleability. Its high ionization enthalpy is closer to that of the halogens, due to its unwillingness to lose its single electron. Like the halogens, hydrogen forms diatomic molecules, compounds with other elements to yield hydrides, and thousands of covalent compounds. In most of its other properties, hydrogen is decidedly less reactive than the halogens.
Because of its peculiar electron configuration and chemical behavior, hydrogen occupies a position in the periodic table that unifies its place as rather special in role and properties concerning chemistry and physics.
Applications and Relevance of Hydrogen Isotopes
Hydrogen isotopes have very wide applications in life, ranging from nuclear fusion to medical imaging and studies on the environment. Protium found its core application in fuel cells and many various industrial processes. Deuterium might be used to set ground for nuclear reactions and scientific experiments. Lastly, tritium finds its place in use for military uses and fusion research. The section tries to expound on how these isotopes enrich technology, bring improvement in medical diagnosis, and lead to solutions to energy challenges.
Recommended topic video on(Isotopes of hydrogen)
Some Solved Examples
Example 1
Question:
Hydrogen is the lightest element with atomic number 1. It exists in nature primarily as:
1) \( H_2 \)
2) \( H_3 \)
3) \( H_4 \)
4) \( H_5 \)
Solution:
Hydrogen exists primarily as \( H_2 \), which is a diatomic molecule. Hence, the correct answer is option (1).
Example 2
Question:
Which of the following statements about deuterium (D) is true?
1) It is a radioactive isotope of hydrogen.
2) It has one proton and two neutrons in its nucleus.
3) It is the most abundant isotope of hydrogen.
4) It has the same chemical properties as protium.
Solution:
Deuterium (D) has one proton and one neutron in its nucleus. It is stable and has similar chemical properties to protium (ordinary hydrogen). Therefore, the correct answer is option (4).
Example 3
Question:
Which isotope of hydrogen is used in nuclear fusion reactions?
1) hydrogen
2) Deuterium (correct)
3) Tritium
4) None
Solution:
Deuterium (D) is used in nuclear fusion reactions due to its ability to readily undergo fusion at achievable temperatures and pressures. Tritium (T), another isotope of hydrogen, can also be used in fusion reactions to enhance the energy output, but it is radioactive and more difficult to obtain. Therefore, deuterium is preferred for practical fusion applications.
Summary
The isotopes of hydrogen: protium, deuterium, tritium -- have been looked upon in general. Their properties and significance for scientific investigations were shown, and possibilities of application-based. Mastering these isotopes will enable a deeper knowledge of the basics of chemistry and prove them to be crucially important for new technologies and solutions to global problems of the world.
Frequently Asked Questions (FAQs)
Some common isotopes of hydrogen come in the form of protium, deuterium, and tritium. Again, these isotopes need the same number of protons and electrons. They have different numbers of neutrons
Protium does not contain neutrons while deuterium contains one neutron, and tritium two neutrons; it is also radioactive. The point here is that isotopes have approximately the same chemical but different physical properties attributed to large mass differences.
Elucidate that deuterium and tritium play a reasonable role in nuclear reactions of fusion in which isotopic properties add huge amounts of energy release.
Deuterium finds relatively wide application in heavy water reactors and pharmaceutical research, and, in fact, in biochemical studies as a tracer due to the stability of techniques with neutron-capturing properties.
It becomes radioactive because of the unstable nucleus of the. It has practical applicability in self-powered lighting devices and nuclear weapons. It is also used for gas tracing in environmental studies.
