Hydrogen - Notes, Topics, Books, FAQs
Hydrogen, the lightest and simplest element discovered to date, is composed of a single proton and a single electron. It exists in three isotopic forms: protium, deuterium, and tritium, each distinguished by the number of neutrons in its nucleus. Hydrogen is also an important part of many biomolecules, such as proteins, DNA, carbohydrates, etc. In nature, it is available as natural gas, but in pure form, it is highly inflammable. In the modern periodic table, its position is not fixed because it belongs to the s-block, but its properties resemble those of non-metals.
This Story also Contains
- Important Topics of Hydrogen
- Various Important Real-life Applications Of Hydrogen:
- Overview Of The Chapter
- Hydrides
- Physical Properties
- Chemical Properties
- Dihydrogen As a Fuel
- How To Prepare For Hydrogen?
- Prescribed Books
- Previous Year Questions Of Hydrogen
- Practice more questions from the link given below:
- Conclusion
Important Topics of Hydrogen
Isotopes of Hydrogen:
Hydrogen has three key isotopes—protium (1H), deuterium (2H or D), and tritium (3H or T). All three share the same single-electron configuration but differ in neutron count: protium has none, deuterium has one, and tritium has two neutrons. Tritium is the only radioactive isotope of hydrogen
Dihydrogen:
Dihydrogen is a colorless, odorless, and tasteless gas that burns readily. It is lighter than air and does not dissolve in water. Since hydrogen’s electronegativity falls between that of metals and non-metals, it can behave both as an electron donor (electropositive) and an electron acceptor (electronegative).
Hydrides:
Hydrides are compounds in which hydrogen is chemically bonded to an element that is more electropositive. These substances usually appear as colorless or grayish crystalline solids and are characterized by high melting and boiling points.
-
Ionic (Saline) Hydrides
-
Formed by reaction of hydrogen with highly electropositive metals (usually s-block).
-
Example: NaH, CaH₂
-
Nature: Ionic (H⁻)
-
Properties:
-
High melting point
-
Reacts with water to liberate hydrogen gas:
$\mathrm{CaH}_2+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2+2 \mathrm{H}_2$
-
-
-
Covalent (Molecular) Hydrides
-
Formed by reaction of hydrogen with p-block elements.
-
Example: CH₄, NH₃, HCl
-
Nature: Covalent
-
Properties:
-
Low melting and boiling points
-
Usually gases at room temperature
-
-
-
Metallic (Interstitial) Hydrides
-
Formed by reaction of hydrogen with transition metals.
-
Example: TiH₁.₈, VH₀.₈
-
Nature: Hydrogen occupies interstitial spaces in the metal lattice.
-
Properties:
-
High melting point
-
Conducts electricity
-
Usually non-stoichiometric
-
-
Importance Of Water (H2O):
Water is known for having solvent capabilities, and colloquially it is often called the "universal solvent" because it dissolves more substances than any other liquid. Water is neutral in nature. pH of the pure water is 7. It is a weak electrolyte and ionizes into H+ and OH- ions.
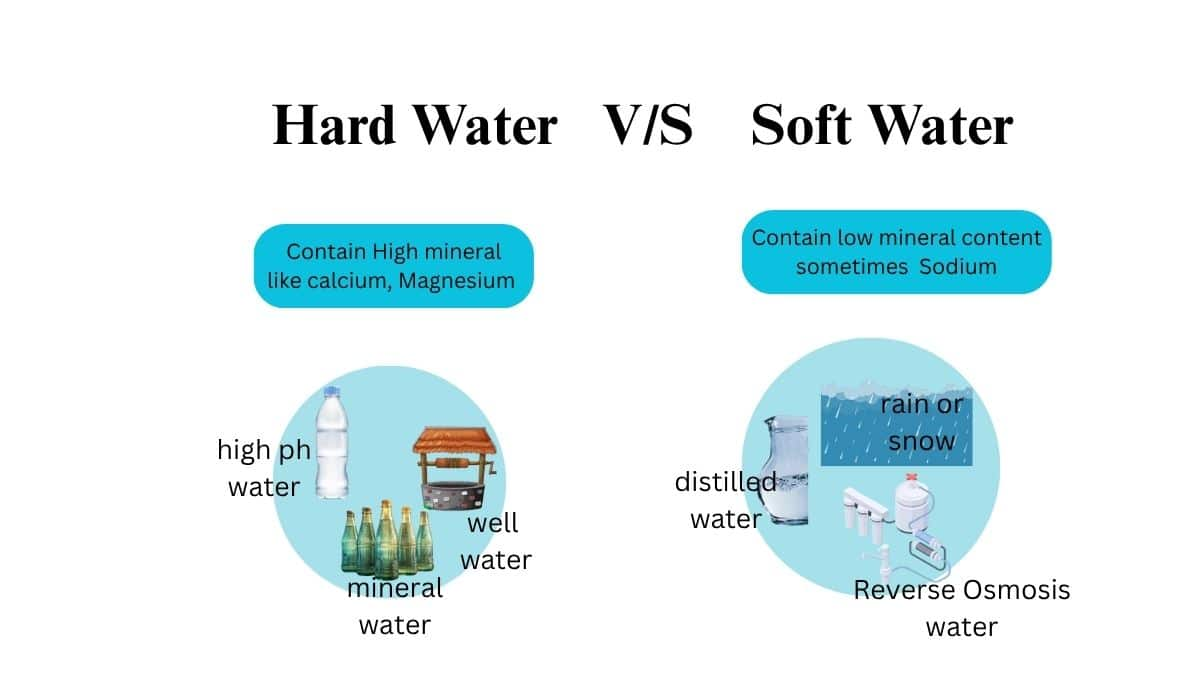
Hard Water And Soft Water:
When water has a high concentration of dissolved minerals—mostly calcium and magnesium and when it produces sufficient lather with soap that is called Hard Water And Soft Water respectively.
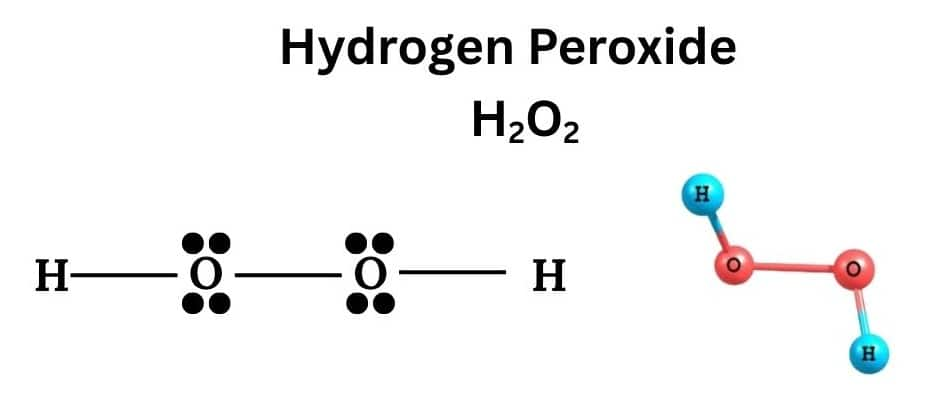
Hydrogen Peroxide:
Hydrogen Peroxide has a formula H2O2 , it is a colorless, unstable, and highly reactive liquid at room temperature. It is also an important chemical used in pollution control treatment of domestic and industrial effluents.
Various Important Real-life Applications Of Hydrogen:
- In petroleum refineries, it is used to remove sulfur content. In these plants, it is also used for hydroisomerization.

- In ultraviolet lamps, it is used in the form of deuterium. These lamps on heating produce light in the ultraviolet region.

- One of the first uses of hydrogen is its use in gas balloons. Due to its light weight, it is used for flying hot balloons.

Overview Of The Chapter
Hydrogen is the simplest and lightest element, commonly found as diatomic H₂ gas. It has three isotopes—protium (1H), deuterium (2H), and radioactive tritium (3H)—which differ only in their neutron count. H₂ is colorless, odorless, tasteless, flammable, lighter than air, and barely dissolves in water. It’s produced in labs (such as by reacting zinc with acid) and on an industrial scale using methods like electrolysis or steam reforming. Hydrogen forms ionic, covalent, and metallic hydrides and is widely used in making ammonia and methanol, hydrogenating oils, reducing metal oxides, fueling rockets and fuel cells, and welding. In this article, you will study the various insights about this chapter and preparation tips.
The Position Of Hydrogen In The Periodic Table:
Hydrogen is placed in the first position of the periodic table. However, its actual position is always has been a matter of discussion in science. Its electronic configuration is 1s1, which means either it requires one more electron to completely fulfill the s orbital or it can lose one electron. Thus Hydrogen behaves like alkali metals and halogens. Like alkali metals, Hydrogen forms halides, oxides, and sulfides. But in terms of ionization enthalpy, Hydrogen resembles more to halogens than alkali metals.
Isotopes Of Hydrogen:
As you all must know from your earlier classes, Hydrogen has three isotopes: protium, deuterium and tritium. Protium has no neutron, deuterium has 1 neutron and tritium has 2 neutrons. Since the electronic configuration of all these isotopes is same therefore chemical properties of these isotopes are same. However, their physical properties are considerably different from each other.
Dihydrogen
Dihydrogen is one of the most important chemicals in the universe. It is the most abundant element in the universe with almost 70% mass of the universe. In this section, you will study the preparation of dihydrogen, its properties, and uses.
- Preparation:
Hydrogen is prepared by the reaction of granulated zinc with hydrochloric acid or with aqueous alkali.
$\\*Zn\: +\: 2H^{+}\: \rightarrow \: Zn^{2+}\:+\: H_{2}\\*Zn\: +\: 2NaOH\: \rightarrow \: Na_{2}ZnO_{2}\: +\: H_{2}$ -
Properties of Dihydrogen
(i) Physical properties:
(a) It is colourless, tasteless, combustible gas.
(b) It is almost 1/4 times lighter than air.
(c) It is very low solubility in water.
(ii) Chemical Properties:
The bond dissociation enthalpy of dihydrogen is very high thus, it is very inert at room temperature. -
Reaction with halogens:
It reacts with halogens and forms hydrogen halides of the form HX.
$H_{2}(g)\: +\: X_{2}(g)\: \rightarrow\: 2HX(g)$ -
Reaction with dioxygen:
It reacts with dioxygen and forms water.
$2H_{2}(g)\: +\: O_{2}(g)\: \rightarrow\: 2H_{2}O(l)$ -
Reaction with dinitrogen:
It reacts with dinitrogen and forms ammonia:
$3H_{2}(g)\: +\: N_{2}(g)\: \rightarrow\: 2NH_{3}(g)$ -
Reaction with metals:
It reacts with metals and forms hydrides
$H_{2}(g)\: +\: 2M(g)\: \rightarrow\: 2MH(s)$ - Uses
(i) It is used in the synthesis of ammonia.
(ii) It is used in the manufacture of vanaspati fat.
(iii) It is used in the production of metal hydrides.
(iv) It is also used for the synthesis of hydrogen chloride.
Hydrides
Water
Water is present on the earth in a tremendous amount. In the human body, it is available up to 70% and in some fruits like watermelon, it is present up to 96%. On earth, it is present in various forms as given in the table:
|
Source |
Total(%) |
|
Oceans |
97.33 |
|
Saline lakes and inland seas |
0.008 |
|
Polar ice and glaciers |
2.04 |
|
Groundwater |
0.61 |
|
Lakes |
0.009 |
|
Soil moisture |
0.005 |
|
Atmospheric water vapour |
0.001 |
|
Rivers |
0.0001 |
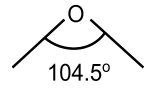
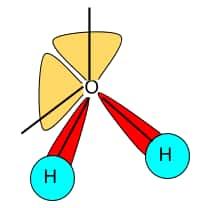
Water has the bent structure with 104.50 bond angle and 2 lone pair of electrons on the oxygen atom. It is a highly polar molecule and forms hydrogen bonding with other water molecules. The structures of water are depicted in fig below:
Physical Properties
- It is a colourless liquid.
- It's melting point is 0oC and boiling point is 100oC.
- It is an excellent solvent for the transportation of ions and molecules.
- It has a high heat of vaporization and high heat capacity .
- It has higher specific heat, thermal conductivity, surface tension, etc.
Chemical Properties
- Amphoteric nature
Water is amphoteric in nature. Thus it has the ability to act as an acid as well as a base. - Redox reactions with water
Water is reduced very easily on reacting with highly electropositive metals.
$2H_{2}O(l)\: +\: 2Na(s)\: \rightarrow \: 2NaOH(aq)\: +\: H_{2}(g)$ - Hydrolysis reaction
Some covalent and ionic compounds are hydrolysed in water.
$\mathrm{P_{4}O_{10}(s)\, +\, 6H_{2}O(l)\, \rightarrow \, 4H_{3}PO_{4}(aq)}$
Hard and Soft Water
When salts of calcium and magnesium are dissolved in the water then it is called as hard water, but when these salts are not present in the water then it is known as soft water. Hard water does not form lather with soaps but soft water forms lather. For washing in hard water, soaps are not efficient and thus we use detergents. With proper water treatment, this hardness of water can be removed.
Hydrogen Peroxide(H2O2)
Hydrogen peroxide is one of the most important compounds which is used in pollution control treatment. Some of the important physical properties of hydrogen peroxide are given in the table below:
|
Melting Point(K) |
272.4 |
|
Boiling Point(K) |
423 |
|
Vapor pressure |
1.9 |
|
Density |
1.64 |
|
Dielectric Constant |
70.7 |
|
Electrical Conductivity |
5.1x10-8 |
- Preparation: Hydrogen peroxide is prepared by the following methods as discussed below:
(i) In this method, barium peroxide is treated with sulphuric acid and thus form barium sulphate and hydrogen peroxide.
$\mathrm{BaO_{2}.8H_{2}O(s)\: +\: H_{2}SO_{4}(aq)\: \rightarrow\: BaSO_{4}(s)\: +\: H_{2}O_{2}(aq)\: +\: 8H_{2}O(l)}$
(ii) In this method, with the help of electrolytic oxidation of acidified sulfate solutions, peroxodisulphate is obtained. This peroxodisulphate is then hydrolyzed to yield hydrogen peroxide.
$\mathrm{2HSO_{4}^{-}(aq)\, \overset{Electrolysis}{\rightarrow}\, HO_{3}SOOSO_{3}H(aq)\overset{Hydrolysis}{\rightarrow}\, 2HSO_{4}^{-}(aq)\, +\, 2H^{+}(aq)\, +\, H_{2}O_{2}}$ - Uses
(i) In daily life, hydrogen peroxide is used as a hair bleach.
(ii) It is used in the synthesis of tartaric acid and some food products.
(iii) It is used a bleaching agent for textiles, leather, fats,etc.
(iv) It is used to manufacture chemicals like sodium perborate and per-carbonate.
Dihydrogen As a Fuel
Hydrogen gas burns with a high energy output, producing nearly three times the energy of petrol per unit mass. It emits fewer pollutants, with nitrogen oxides being the primary byproduct. This issue can be mitigated by introducing water into the combustion process, lowering the temperature and reducing nitrogen oxide formation.
However, using hydrogen as a fuel presents challenges. Compressed hydrogen cylinders are significantly heavier than petrol tanks, approximately 30 times more. Additionally, liquefying hydrogen requires cooling it to around 20 K, necessitating expensive, insulated storage tanks.
How To Prepare For Hydrogen?
-
Understand the Basics
Before diving into this chapter, ensure you're familiar with the "Periodic Classification of Elements" as it provides foundational knowledge essential for understanding hydrogen's position and behavior. -
Focus on Theory
This chapter is entirely theory-based, making it relatively straightforward. There's no need to memorize complex formulas; instead, concentrate on grasping the concepts and their applications. -
Practice Regularly
While the chapter is theory-heavy, practicing related numerical problems can enhance your understanding and retention of the material. -
Stay Consistent
Regular study sessions, even if brief, can be more effective than cramming. Consistency helps reinforce learning and improves long-term retention. -
Use Visual Aids
Diagrams, charts, and tables can aid in better understanding and memorization of key concepts, such as the preparation methods and properties of hydrogen. -
Clarify Doubts Promptly
Don't hesitate to ask teachers or peers about concepts you find challenging. Clearing doubts promptly ensures a solid grasp of the subject matter. -
Relate to Real-World Applications
Understanding the practical applications of hydrogen, like its use in fuel cells or the Haber process, can make the theoretical concepts more relatable and easier to comprehend.
Also Read,
Prescribed Books
First, you must finish the class XI and XII NCERT textbook and solve each and every example and unsolved question given in it. Then for advanced level preparation like JEE and NEET, you must follow O.P. Tandon. You must definitely solve the previous year's papers. Meanwhile, in the preparation, you must continuously give the mock tests for the depth of knowledge. Our platform will help you with a variety of questions for deeper knowledge with the help of videos, articles and mock tests.
Previous Year Questions Of Hydrogen
Question 1: $\mathrm{O}-\mathrm{O}$ bond length in $\mathrm{H}_2 \mathrm{O}_2$ is $\underline{\mathrm{X}}$ than the $\mathrm{O}-\mathrm{O}$ bond length in $\mathrm{F}_2 \mathrm{O}_2$. The $\mathrm{O}-\mathrm{H}$ bond length in $\mathrm{H}_2 \mathrm{O}_2$ is $\underline{Y}$ than that of the $\mathrm{O}-\mathrm{F}$ bond in $\mathrm{F}_2 \mathrm{O}_2$.
Choose the correct option for $X$ and $Y$ from those given below:
1) X-shorter, Y -longer
2) X-shorter, Y-shorter
3) X-longer, Y-shorter
4) X-longer, Y-longer
Solution: 
$\rightarrow(\mathrm{O}-\mathrm{O}) \mathrm{BL}$ in $\mathrm{H}_2 \mathrm{O}_2$ in longer then $(\mathrm{O}-\mathrm{O}) \mathrm{BL}$ in $\mathrm{O}_2 \mathrm{~F}_2$
$\rightarrow(\mathrm{O}-\mathrm{H}) \mathrm{BL}$ in $\mathrm{H}_2 \mathrm{O}_2$ in shorter than $(\mathrm{O}-\mathrm{F}) \mathrm{BL}$ in $\mathrm{O}_2 \mathrm{~F}_2$
Hence, the answer is the option (3).
Question 2: Which of the following silicates are used as ion exchangers in softening of hard water:
1) Orthosilicate
2) Zeolite
3) Cyclic silicate
4) Chain silicate
Solution:
Zeolites are 3-D silicates. These are used as ion exchangers in softening of hard water common zeolite is sodium aluminosilicate.
Hence, the answer is option (2).
Question 3: Which of the following statements is correct?
(i) Metallic hydrides are deficient of Hydrogen.
(ii) Metallic hydrides conduct heat and electricity.
(iii) Ionic hydrides do not conduct electricity in solid-state.
(iv) Ionic hydrides are very good conductors of electricity in solid-state.
1) (i), (iii), and (iv)
2) (ii), (iii), and (iv)
3) (i), (ii), and (iii)
4) (i), (ii), and (iv)
Solution:
The answer is the option (i), (ii), and (iii) Metallic hydrides are deficient of Hydrogen, Metallic hydrides conduct heat and electricity, and Ionic hydrides do not conduct electricity in solid-state.
Hydride and not volatile or conductive in the solid-state but are crystalline instead. While metallic hydrides are non-stoichiometric hydrides. They only conduct electricity in their molten state.
Hence, the answer is the option (3).
Practice more questions from the link given below:
Conclusion
Hydrogen is the simplest element, made of one proton and one electron, and has three isotopes: protium, deuterium, and tritium. It normally exists as diatomic H₂ gas, which has two nuclear spin forms (ortho and para) and shows traits of both alkali metals and halogens. Hydrogen is prepared in labs (e.g., metal-acid reactions) and industrially (steam reforming). It forms ionic, covalent, and metallic hydrides. Key uses include manufacturing ammonia and methanol, hydrogenating oils, reducing metal oxides, fuel cells, rockets, welding, and supporting water and hydrogen peroxide chemistry.
Frequently Asked Questions (FAQs)
Hydrogen has three main isotopes:
- Protium (¹H): The most common isotope, with one proton and no neutrons.
- Deuterium (²H or D): Contains one proton and one neutron. It is also called "heavy hydrogen."
- Tritium (³H or T): Contains one proton and two neutrons. It is radioactive.
Hydrogen occupies a unique position in the periodic table. Although it is placed in Group 1 with alkali metals due to its tendency to lose an electron (forming H⁺ ion), it also shares properties with halogens (Group 17) as it can gain an electron to form a hydride ion (H⁻). Furthermore, it exists as a diatomic molecule (H₂) at room temperature, unlike many alkali metals.
IIIT Kota is the top engineering choice for academic rigor and placement. Government Medical College stands out for MBBS at reasonable fees. RTU Kota provides diverse technical courses at a state-level university cost. For budget-conscious choices, GIET, MIT, and RN Modi College offer solid engineering programs at lower fees.
Hydrogen atoms pair up to form H₂ because sharing two electrons fills their 1s shells, making the molecule more stable—much like achieving a helium-like configuration.
Like alkali metals, it has a 1s¹ electron configuration and forms H⁺ ions. Like halogens, it needs one electron to complete its shell and forms H⁻ or covalent bonds.
Yes, hydrogen can power vehicles equipped with fuel cells, which convert hydrogen and oxygen into electricity to power electric motors. Hydrogen fuel cell vehicles (FCVs) offer a zero-emission alternative to traditional gasoline or diesel vehicles.
Hydrogen can be considered a clean energy source when produced through renewable methods, such as electrolysis powered by solar or wind energy. Fuel cells emitting only water vapor as a byproduct also contribute to reducing greenhouse gas emissions. However, the environmental impact depends on the production method used.
Hydrogen has numerous applications, such as:
- Fuel Cells: Providing clean energy for vehicles and stationary power generation.
- Industrial Processes: Serving as a feedstock in the production of ammonia, petroleum refining, and food processing.
- Energy Storage: Storing excess renewable energy and supplying it when needed.
- Metal Processing: Used in various metallurgical processes to remove impurities.
Hydrogen can be produced through various methods, including:
- Steam Methane Reforming (SMR): A process that extracts hydrogen from natural gas.
- Electrolysis: Splitting water into hydrogen and oxygen using electricity.
- Gasification: Converting organic or fossil-based materials into hydrogen and other products.
- Biomass Gasification: Producing hydrogen from organic materials through thermal or chemical means.
Questions related to
On Question asked by student community
Hello Jitendra. Chapters like Surface Chemistry, Hydrogen and Chemistry in everyday life are not in the neet 2026 syllabus so it will be better for you to completely skip these chapters. If you want to stay on the safer side then you can take an overview of important topics from
Dear aspirant,
When an electron transitions from the fifth orbit to the ground state in a hydrogen atom, a total of 10 spectral lines can be observed. This is because the electron can make transitions to any lower energy level, including directly to the ground state (n=1), or to intermediate
Correct Answer: 1:4
Solution : The correct option is 1:4.
If the density of oxygen is 16 times that of hydrogen, the ratio of their velocities of sound will be the same as the ratio of their densities
The velocity of sound from oxygen to hydrogen is 1:4.
v=√γP/ρ.
Correct Answer: between 4 and 7
Solution : The correct choice is the fourth option.
Explanation: According to the passage, the pH value of human skin is generally in a range between 4.0 and 7.0, depending on various factors like body parts, age, genetics, ethnicity, and environmental conditions.
Correct Answer: Neither I nor II follows
Solution : According to the above-given statement –
Conclusion I: North Korea will declare a state of war against the US and its ally countries soon – Though North Korea has successfully tested a hydrogen bomb, it does not imply that North Korea
