Salt bridge
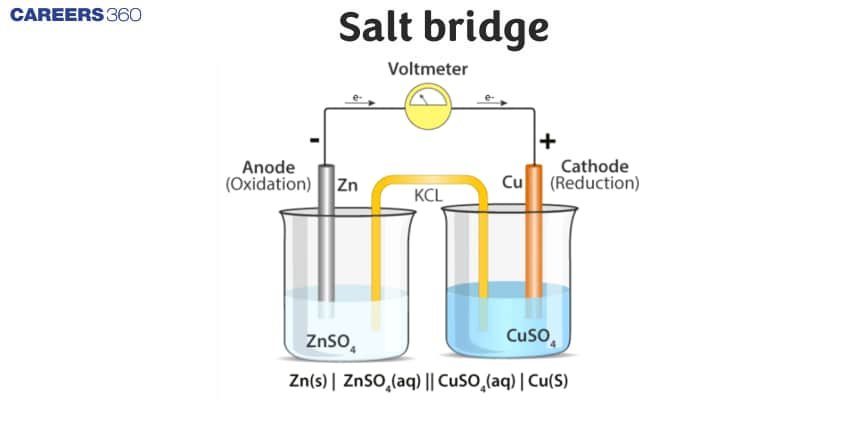
A salt bridge is a U-shaped tube containing a concentrated solution of an electrolyte like KCl, KNO3, K2SO4, etc. or a solidified solution of such an electrolyte in agar-agar and gelatine An inert electrolyte is one whose ions do not take part in redox reaction and also does not react with electrolyte used.
This Story also Contains
- Salt Bridge And Its Conditions
- The function of a Salt Bridge
- Conditions for salt bridge
- Some Solved Example
- Summary
Salt Bridge And Its Conditions
It maintains electrical neutrality in two compartments by allowing the movement of anions toward the anodic compartment and cations toward the cathodic compartment.
- It is a glass tube having KCl, KNO3, and ammonium nitrate in a gelatin gel or agar-agar paste.
- The gelatin gel allows ionic movement through it but prevents any kind of mixing.
- In the case of KCl or ammonium nitrate, the ionic mobility of cation and anion are the same.
The function of a Salt Bridge
- A salt bridge acts as an electrical contact between the two half-cells.
- It prevents the mechanical flow of solution but it provides a free path for the migration of ions to maintain an electric current through the electrolyte solution. It prevents the accumulation of excess charges.
- A salt bridge helps maintain the charge balance in the two half-cells.
- A salt bridge minimizes/eliminates the liquid junction potential.
- To complete the electrical circuit by allowing the ion to flow from one solution to the other without mixing the other solution
Conditions for salt bridge
A salt bridge is crucial for maintaining electrical neutrality in electrochemical cells.
- Electrolyte Solution- It must contain a strong electrolyte, such as potassium chloride (KCl) or sodium sulfate (Na2SO4), which dissociates completely in solution.
- Electrolyte Concentration- The concentration should be high enough to provide a sufficient ionic flow but not so high that it causes precipitation.
- Inert Material- The salt bridge should be made from an inert material that does not react with the electrolytes in the cell.
- Conductivity- It should be designed to allow for the easy flow of ions between the two half-cells.
- Porosity- If it's a physical salt bridge, it should have a porous structure to allow ion movement while preventing the mixing of the two solutions.
- Stability- The bridge should be stable and not degrade or dissolve rapidly in the electrolyte solutions.
Recommended topic video on (salt bridge)
Some Solved Example
Example.1
1. A saturated solution of KNO3 is used to make a 'salt bridge' because:
1)velocity of K+ is greater than that of NO-3
2)velocity of NO-3 is greater than of K+
3) (correct)velocity of both NO-3 and K+ are nearly the same
4)KNO3 is highly soluble in water
Solution
The salt bridge possesses the electrolyte having nearly the same ionic mobilities as its cation and anion.
Hence, the answer is the option (3).
Example.2
2. Which of the following statements is true about the salt bridge?
1)The ionic mobility of the ions in the salt bridge should be vastly differing in value.
2) (correct)The ions in the salt bridge must be inert to the ions of the cell.
3)The ions in the salt bridge must be reactive to the ions of the cell
4) A salt bridge provides an electric contact between the two solutions by allowing them to mix.
Solution
The salt bridge provides electric contact between the two solutions without allowing them to mix.
The ions in the salt bridge must be inert to the ions of the cell.
Therefore, the correct option is (2).
Example.3
3. A current of 5 amp is passed for one hour through an aqueous solution of copper sulfate using copper electrodes. What is the change in the mass of the cathode?
1)3.17
2) (correct)5.92
3)4.92
4)5.72
Solution
The reaction occurring at the given cathode is
Cu2++2e−→Cu (Cathode
Thus, 1 mole of Cu is deposited by passing 2 moles of electrons.
So, the charge required to deposit 1 mole of Cu=2×96500=193000C
Now,
The actual charge passed through the electrode 5×60×60=18000
So, moles of Cu deposited =18000/193000
Thus, the mass of copper deposited =18000/193000×63.5=5.92 g
Hence, the answer is (5.92).
Example.4
4. If hydrogen electrodes dipped in two solutions of pH = 3 and pH = 6 are connected by a salt bridge, the EMFcell (in V) is:
1)0.052
2)0.067
3)0.277
4) (correct)0.177
Solution
For the given concentration cell:
H2|H−(c1)‖H+(c2)|H2Ecell =−0.059(pHc−pHa)=−0.059(3−6)=0.1773 V
Cell will be feasible only when [c2]c>[c1]a or pHc<pHa
Hence, the answer is the option (4).
Summary
Salt bridge prevents the charge built up to stop the redox reaction and ensures that the operation or response is continuous and effective. Salt bridges are also but very rarely used in the electrochemical cell where they help maintain the proper ionic movement needed for electrolysis.
