Structures of Beryllium Chloride in the Solid State and Vapour Phase
Beryllium chloride (BeCl₂), an inorganic material, is an essential chemical reagent in numerous processes. The beryllium ion (Be2+) is well-known for its unique structure and properties because of its small size and high charge density. The inorganic compound beryllium chloride (BeCl₂) has unique structural characteristics depending on the phase it is in.
This Story also Contains
- Structure of Beryllium Chloride
- Solved Examples
- Conclusion
Solids Phase:
In its solid state, beryllium chloride forms a polymeric chain structure. Every beryllium atom is tetrahedrally linked to four chlorine atoms, while every chlorine atom connects two beryllium atoms. Consequently, BeCl₄ tetrahedra are linked by a one-dimensional chain consisting of common chloride ions. The bonding can be described as ionic with significant covalent character due to the Be3⁺ ions small size and high charge density.
Gas Phase:
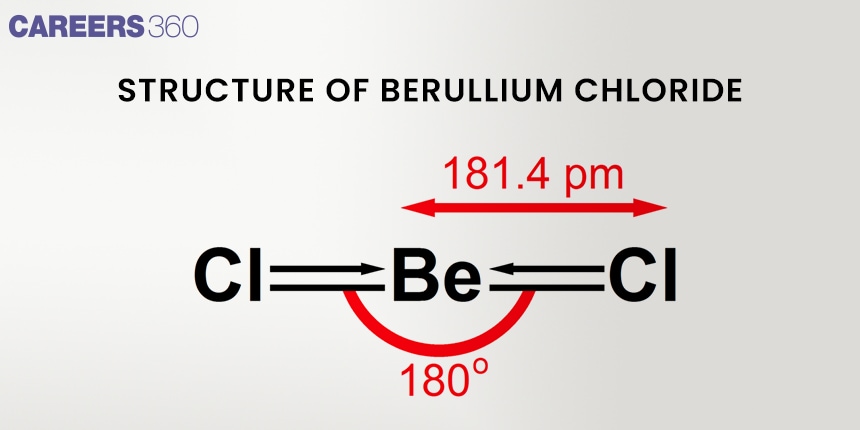
In the gas phase, beryllium chloride exists as distinct BeCl₂ molecules. Each BeCl₂ molecule assumes a linear shape with a 180° bond angle, which is indicative of sp hybridization of the beryllium atom. Covalent bonds make up the bulk of a molecule's bonding.
In this article, we will cover the topic of the structure of beryllium chloride). This topic falls under the broader category of (The s - Block elements), which is a crucial chapter in (Class 11 Chemistry).
Structure of Beryllium Chloride
- All metals on heating with halogens from halides of type MX2M+X2→MX2
- BeCl2 cannot be prepared in aqueous solution due to the formation of hydrated ion [Be(H2O)4]2+.
- BeCl2 can be prepared by heating BeO in the presence of coke in a current of chlorine gas.
- Structure of BeCl2
- In Solid State:

- In Liquid State: Two forms of BeCl2 exist at different temperature. Above 1200K, a monomeric form exists as shown below.

Below 1200K, dimeric form exists as shown below.
- In Solid State:
A "covalent alkaline earth metal halide" refers to a compound composed of an alkaline earth metal (Group 2 elements like beryllium, magnesium, calcium, etc.) and a halogen (Group 17 elements like fluorine, chlorine, bromine, iodine, etc.) in which the bonding involves the sharing of electron pairs between the metal and the halogen atoms, indicating a covalent bond.
These compounds typically form when the electronegativity difference between the metal and halogen atoms is not significant enough to warrant a complete transfer of electrons, leading to a shared electron pair between the atoms. Examples include beryllium fluoride (BeF2), magnesium chloride (MgCl2), and calcium bromide (CaBr2). These compounds often have high melting and boiling points due to the strong directional covalent bonds between the metal and halogen atoms, and they may exhibit some degree of solubility in polar solvents due to their polar nature.
Recommended topic video on (Structure of Beryllium Chloride )
Solved Examples
Example:1
Q 1. The structure of beryllium chloride in the solid state and vapour phase, respectively, are :
1) Chain and chain
2) Dimeric and dimeric
3) Chain and dimeric
4) Dimeric and chain
Solution-
As we learned-
Structure of Beryllium chloride in vapour and solid phase. -
A. In vapour state, it exists as a chlorine bridge dimer.
B. In the solid state, BeCl2 has a polymeric structure with chlorine bridges.
BeCl2 is an electron-deficient compound with sp hybridization. Therefore in the solid state, it combines with another BeCl2 molecule to give Be2Cl4 by forming a coordinate bond between Cl and Be thus attaining more stability
However, in the gaseous state, it exists as a single molecule. At room temperature BeCl2 is a polymeric solid, this is because it can form coordinate bonds with other molecules of the same type and forms a chain-like structure.
Therefore, option(3) is correct.
Example :2
Q.2 The covalent alkaline earth metal halide (X=Cl, Br,I) is:
1) MgX2
2) CaX2
3) BeX2
4) SrX2
Solution-
As we learned
Covalent alkaline earth metal halide:-
In chemical terms, all of the alkaline earth metals react with the Halogens to form the alkaline earth metal halides, all of which are ionic crystalline compounds except for Beryllium halide, which is a covalent [exception. BeCl2].
A chain type of structure can be observed in the solid form of beryllium chloride.
These beryllium halides can be generally dissolved in organic solvents.
Hence, the answer is the option (3).
Conclusion
Beryllium chloride (BeCl₂) is a useful chemical for competitive examinations and applications in chemistry since it possesses distinct structural features in different phases. In the solid state, BeCl₂ forms a polymeric chain structure when beryllium atoms are linked to create a one-dimensional chain with significant covalent character. Each beryllium atom has a tetrahedral coupling to four chlorine atoms. BeCl₂ is observed as discrete linear molecules with sp hybridization and predominantly covalent bonding in the gas phase. The hydrated ion [Be(H₂O)₄]²⁺ is produced during the heating of BeO in the presence of coke and chlorine gas, rendering the production of BeCl₂ in an aqueous solution unfeasible. BeCl₂ exists as a monomeric form above 1200K and as a dimeric form below 1200K.
Frequently Asked Questions (FAQs)
Yes, it is. The covalent nature of beryllium chloride lets it dissolve in organic solvents. Several advantages are accrued thereby.
Above 1200 K, BeCl₂ is a monomer but at temperatures below it, the chlorine bridging that takes place changes it into dimers. These points mean structural stability.
BeCl₂ is covalent with appreciable solid-state ionic character. In the vapor phase, it is essentially covalent.
Beryllium chloride is prepared by heating beryllium oxide BeO along with coke in the presence of chlorine gas. Coke helps in the formation of the compound.

