Suspension - Definition, Examples, Types, Properties, FAQs
Have you ever noticed muddy water after it rains or a mixture of flour and water left undisturbed? Why do the solid particles float for a while and then slowly settle at the bottom? And have you wondered how some mixtures appear cloudy while others remain clear? You may have noticed Shake well before use on juice or many medicine bottles. So the answers to all these questions are suspension. It is a mixture where small particles are mixed in a liquid, but they remain undissolved. If the mixture is set aside for some time, the particles settle at the bottom of the container.
This Story also Contains
- Suspension
- Example of Suspension
- Types of Suspension
- Pharmaceutical Suspension
- Properties of Suspension
- True Solution
- Colloids
- Tyndall Effect
- Difference between a colloid and a suspension
- Some Solved Examples
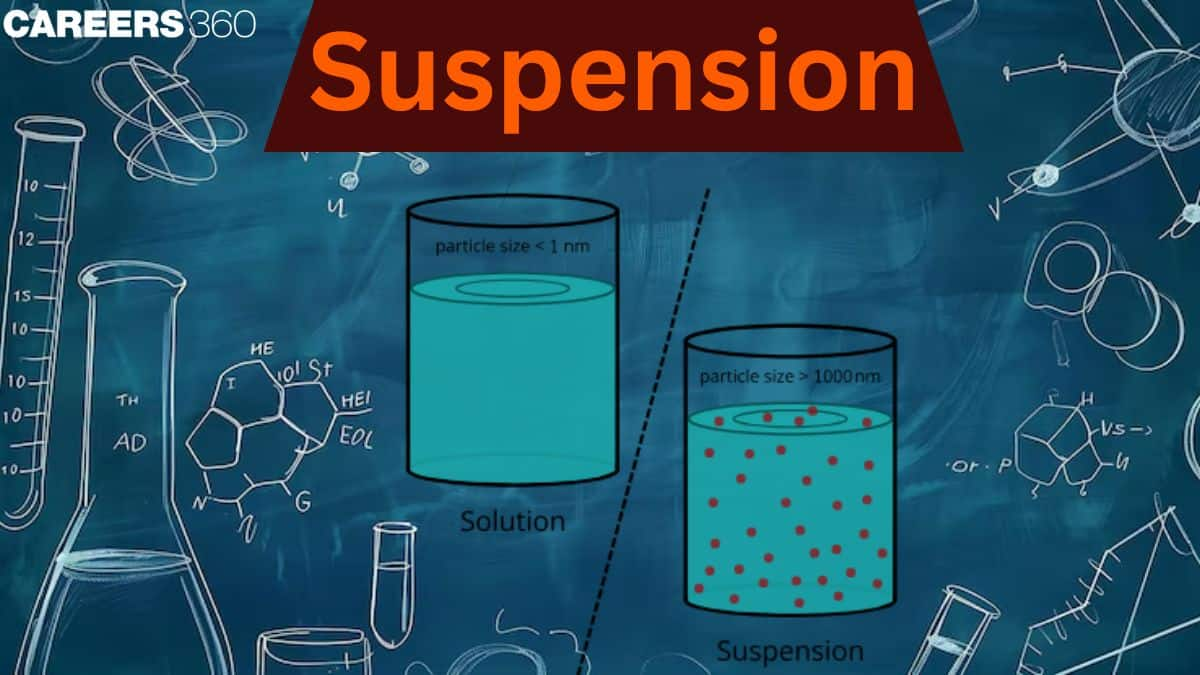
We notice the phenomenon of suspension every day around us, like your favourite chocolate milk, where chocolate settles at the bottom of the container after some time, orange juice with pulp, muddy water, and lemonade with lemon pulp. It is a heterogeneous mixture in which solute particles don't dissolve but rather remain suspended in the medium's bulk. The size of particles in the suspension solution is at least 100 times that of the solution particles.
Suspension
A suspension is a heterogeneous mixture in which solid particles are dispersed throughout a liquid or gas but are not dissolved. These solid particles are large enough to be seen by the naked eye or under a microscope, and they tend to settle at the bottom of the container over time if left undisturbed. The solid particles in a suspension are generally larger than 100 nanometers in size, making them visible to the naked eye or under a microscope. Over time, the solid particles will settle to the bottom of the container due to gravity, which distinguishes suspensions from colloids, where particles remain dispersed.
Example of Suspension
Suspension is commonly used in:
- Flour and water mixture
- Chalk and water mixture
- Muddy water mixture
- Sand and water mixture
- Water-based paints
- Slaked lime (calcium hydroxide) mixed in water
- Magnesium hydroxide and water mixture (Milk of Magnesia) .
Types of Suspension
Oral Suspension
The oral suspension means suspension for oral delivery, made up of undissolved particles of one or more medicinal substances combined with a liquid carrier
Flocculated Suspension
A flocculated suspension is one in which the suspension's particles have been flocculated. A flocculated suspension is made up of big particles (flocks) that cause fast sedimentation. Sedimentation is the process of aggregates or suspended particles settling to the bottom of a liquid. Particles clump together to form massive aggregates that can act as large individual particles. A significant number of particles settle down when these aggregates settle down. Then the sedimentation rate is high. Floccules are the name for these aggregates. Under the influence of gravity, floccules can settle faster than smaller particles.
Deflocculated Suspension
A deflocculated suspension is one in which there has been no flocculation. Single particles take on the role of individual particles in this scenario. These tiny particles sink when sedimentation occurs. Dispersed particles exist as distinct entities in a deflocculated suspension. The sedimentation rate is slow because tiny particles settle more slowly than huge floccules. In comparison to a flocculated suspension, the resulting sediment has a modest volume. The supernatant of this suspension will remain cloudy even after the sediment has formed. Caking is a term used to describe the sediment development in this area.
Difference between flocculated and deflocculated suspension
|
Flocculated suspension
|
Deflocculated suspension
|
| Pleasant appearance due to particle dispersion that is homogeneous. | Sediment is a little unattractive. |
| The supernatant is still hazy. | The supernatant is visible. |
| Particles are self-contained entities. | Particles clump together to form loose aggregates. |
| Since the particles are tiny, the rate of sedimentation is sluggish. | Since flocs are a cluster of tiny particles with a larger size, the rate is high. |
| Particles settle separately and independently. | Particles form flocs as they settle. |
| The sedimentation is densely packed, resulting in a firm cake. | Sediment is a loosely packed network that cannot form a firm cake. |
| It is impossible to re-disperse the hard cake. | The sediment is easily redistributed. |
| Due to the increased specific surface area, bioavailability is higher. | Due to the tiny specific surface area, bioavailability is low. |
Pharmaceutical Suspension
A pharmaceutical suspension is a finely split insoluble substance suspended in a liquid medium in a coarse dispersion of biphasic liquid dose form.
Types of Pharmaceutical Suspension
- The different types of pharmaceutical suspension preparations are suspensions, mixes, magmas, gels, and lotions. The insoluble material scattered in a liquid is referred to as a simple suspension. As stability is considered, dry medication manufacturing appears to be the best option. Before being administered, they are reconstituted as suspensions in an appropriate vehicle. Ex- Antibiotic amoxicillin dispersible pills, Procaine penicillin G powder.
- Gel: Small inorganic particles floating in a liquid medium make up gels, which are semisolid systems. It's made up of a web of microscopic,c discrete particles. It's a two-phase process. Ex-Aluminum hydroxide gel.
- Lotions: Lotions are liquid solutions designed to be applied to undamaged skin without causing friction. Ex- Calamine lotion, hydrocortisone lotion.
- Milks and Magmas: Magmas and milk are aqueous suspensions of insoluble, inorganic medicines that differ mostly from gels in the size of the suspended particles. As they are thick and viscous when produced, they do not require the addition of a suspending agent. Ex-Bentonite magma, milk of magnesia.
- Mixture: Oral liquids having one or more active substances that have been dissolved, suspended, or dispersed in a suitable vehicle are known as mixtures. Standing causes suspended particles to separate slowly, whereas shaking easily redisperses them. Ex- kaolin-pectin mixture.
Properties of Suspension
Suspension features and general characteristics are described below -
- The mixture is heterogeneous.
- Suspension mixture of constituent particles can be seen with the naked eye.
- The particle size in suspension is greater than 100nm.
- Suspension demonstrates the Tyndall effect. It means that particles in a suspension scatter a light beam travelling through it, revealing its route.
- If the particles in a suspension are not disturbed, they settle down. It demonstrates that suspension of the mixture is unstable. The suspension has no Tyndall effect in this situation. Filtration can separate the constituent particles of a suspension.
True Solution
The solvent is the component of the solution that dissolves the other component, whereas the solute is the component that is dissolved in the solvent. In general, the amount of solute in a solution is smaller than the amount of solvent.
Solution Examples
- Sugar and water mixture
- Iodine tincture (solution of iodine in alcohol)
- A glass of soda water
- Oxygen (a homogeneous solution of various gases)
- Alloys (Combination of two or more metals, or a metal and a non-metal that can't be separated by physical means.) Ex-Stainless Steel, Brass etc.
Properties of True Solution
The following properties are:
- The mixture is homogeneous.
- The particles in the solution have a diameter of less than 1 nm.
- Simple physical separation methods, such as filtration, cannot separate solution particles.
Types of True Solutions
Depending upon the amount of solute present in a solution, it can be classified as dilute, concentrated, or a saturated solution. A solution that contains a relatively larger amount of solute is a concentrated solution, while a solution that contains a relatively smaller amount of solute is a dilute solution.
Colloids
A colloid is a heterogeneous mixture in which particles are evenly distributed throughout the fluid. It is also known as a colloidal solution. The term colloid is occasionally used to refer to the dispersed component in a colloidal solution alone, but colloidal suspension clearly refers to the entire mixture. Despite the fact that suspension and colloidal suspension (solution) are both forms of mixtures. Because of the smaller particle size than the suspension, it seems to be homogeneous, and the Tyndall effect is also demonstrated.
Colloidal Properties
- A colloid is a heterogeneous mixture with colloidal particles that are invisible to the human eye.
- Dispersed phase particles in colloids have a diameter of 1–100 nm (approximately).
- They demonstrate Tyndall's effect.
- When dispersed particles in colloids are left undisturbed, they do not settle down.
- Colloids are mixes that are stable.
- The dispersed phase and the dispersion medium in colloids cannot be separated by filtration.
- Brownian movement is shown by the dispersed particles.
Also read :
- NCERT notes Class 11 Chemistry Chapter 5 States of Matter
- NCERT solutions for Class 11 Chemistry Chapter 5 States of Matter
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 5 States of Matter
Components of a colloidal solution
A colloidal solution comprises two components: the dispersed phase and the dispersing medium. The dispersed phase of a colloidal solution is the solute-like component, while the dispersing medium is the solvent-like component. Solid, liquid, or gas can be used as the dispersed phase and dispersing medium.
Forms of colloidal solution
Colloids are divided into two categories based on their constituents: dispersed phase and dispersing medium.
- Aerosol
- Liquid Aerosol
- Solid Aerosol
- Foam
- Emulsion
- Sol
- Solid foam
- Gel
- Solid sol
Gas as a dispersing medium
|
Related Topics link, |
Aerosol: Aerosol is a mixture that is formed when solid or liquid particles are dispersed in a gaseous medium. For instance, cloud, smog, and smoke.
Liquid and solid aerosols are the two forms of aerosols.
- Liquid Aerosol: This mixture is termed as Fog, mist, hair spray, and other similar effects are examples.
- Solid Aerosol: Solid Aerosol is a mixture in which solid particles are in the dispersed phase and gas is in the dispersing phase. Smoke, air particles, vehicular exhaust, and so forth.
Liquid as a dispersing medium
- Foam: When a liquid acts as a dispersion medium and a gas acts as a dispersing medium, the mixture is termed foam. For instance, shaving cream, soap bubbles, and so forth.
- Emulsion: Emulsion is a colloidal solution in which the dispersing medium and the dispersed phase are both liquids. Ex-Milk, butter, face cream.
- Sol: When liquid is the dispersion medium and solid is the dispersed phase, the colloidal solution is called sol. For instance, blood, ink, paint, and so on.
Solid as a dispersing medium
- Solid foam: Solid foam is created by combining solid as the dispersing medium and gas as the dispersed phase. Ex- Styrofoam, pumice stone, bread, and so on.
- Gel: Gel is created by combining a solid as a dispersing medium with a liquid as the dispersed phase. Ex-Gelatin, jelly, hair gel, and so on.
- Solid sol: Solid sol is created by combining a solid as a dispersing medium with a solid as a dispersed phase.
|
Dispersed medium |
Dispersed phase |
Type |
Phase |
|
Gas
| Liquid | Aerosol | Fog |
| Liquid | Gas | Aerosol | Smoke |
| Liquid | Gas | Foam | Shaving cream |
| Liquid | Liquid | Emulsion | Milk |
| Liquid | Solid | Sol | Mud |
| Solid | Gas | Foam | Sponge, cake |
| Solid | Liquid | Gel | Cheese |
| Solid | Solid | Solid sol | Colored gemstone, glasses |
Tyndall Effect
It can be used to determine if the solution is a colloid. When a beam of light is passed through a colloid, it is not allowed to completely pass through the colloidal particles present in the solution. When compared to red light, blue light is scattered to a greater extent. This is due to the fact that blue light has a shorter wavelength than red light. This is why the smoke emitted by motorcycles might appear blue at times.
Examples of the Tyndall Effect
The Tyndall Effect can be seen in milk, which is a colloid that contains fat and protein globules. In a foggy environment, when a torch is turned on, the light's path becomes visible.
Difference between Solution Suspension and Colloids
|
Property
|
Suspension
|
Colloids
|
Solution
|
|
Particle size
| > 100 nm | Between 1 to 100 nm | < 100 nm |
| Homogeneous/ Heterogeneous | Homogeneous | Homogeneous | Homogeneous |
| Tyndall Effect | Shows effect | Shows | Does not show |
| Brownian movement | May show | Shows | Do not show (mostly) |
| Appearance | Opaque | Transparent | Transparent |
| Settling of particles | Settles on their own | Settle on centrifugation | Do not settle |
| Method of separation | Can be separated by physical methods such as filtration | Cannot be separated by physical methods | Cannot be separated by a physical method |
| Stability | Unstable | Stable | Stable |
| Examples | Flour and water mixture | Smoke, cheese | Sugar and water solution |
Difference between a colloid and a suspension
|
Colloids
|
Suspension
| |
|
Type
| It is homogeneous. | It is heterogeneous. |
| Particle size of suspension | The size of the particle is between 2 nm to 1000 nm. | The size of the particle is larger than 1000 nm. |
| Visibility | The particle can’t be seen by a low microscope | The particle can be seen with a low microscope |
Also read
Some Solved Examples
Question 1: Which of the following characteristics is NOT true for a suspension?
A) The particles in a suspension are uniformly distributed.
B) The particles in a suspension can be seen with the naked eye.
C) Suspensions exhibit the Tyndall effect.
D) The particles in a suspension settle down when left undisturbed.
Solution:
Suspensions are heterogeneous mixtures, meaning their particles are not uniformly distributed. They tend to settle down over time, and the larger particles can be seen with the naked eye.
Hence, the correct answer is option A) The particles in a suspension are uniformly distributed.
Question 2: What is the size range of the particles in a suspension?
A) 0.1 nm to 1 nm
B) 1 nm to 100 nm
C) 100 nm to 1 µm
D) Greater than 1 µm
Solution:
The particles in a suspension are typically greater than 1 µm in size. They are large enough to be seen by the naked eye and tend to settle down over time.
Hence, the correct answer is option D) Greater than 1 µm
Question 3: Which of the following methods can be used to separate the particles from a suspension?
A) Filtration
B) Distillation
C) Chromatography
D) Evaporation
Solution:
Filtration is the method used to separate the larger particles of a suspension from the liquid, as the particles are large enough to be trapped by a filter.
Hence, the correct answer is option A) Filtration
Practice More Questions With The Link Given Below
| Colloids practice question and MCQs |
| Peptization(Physical Method of Preparation of Colloids) practice question and MCQs |
Frequently Asked Questions (FAQs)
A suspension is a heterogeneous mixture in which solid particles are dispersed throughout a liquid or gas but are not dissolved. Over time, the solid particles tend to settle at the bottom, resulting in a separation of the mixture. Common examples include muddy water and salad dressings that separate when left standing.
Viscosity helps in maintaining suspension by influencing how the solid particles interact with the liquid. Greater viscosity helps in preventing sedimentation by providing more resistance against the motion of particles, allowing them to remain suspended for a long time.
The main difference between them is the size of solute particles. In solutions, there are micro particles that dissolve completely in the solvent, forming a homogeneous solution, while in suspension, the size of solute particles is very large and the particles do not dissolve in the solution, making the heterogeneous mixture.
Some examples of suspensions are:
- Pulp of lemon in lemonade
- Muddy water
- Orange pulp in orange juice
- Some medicinal syrups
- Paints
Methods of separation of suspensions are:
- Filtration: The mixture is passed through a filter that captures the solute particles.
- Sedimentation: Allows particles to settle at the bottom, after which liquid can be poured off.
Questions related to
On Question asked by student community
Correct Answer: Stagnation in economic development
Solution : The immediate consequence of the suspension of Five-year Plans in India was stagnation in economic development, as the planning process was integral to the country's development strategy.
Correct Answer: It hindered long-term economic planning.
Solution : A major criticism of the suspension of Five-year Plans in India was that it hindered long-term economic planning, disrupting the systematic approach to development that the plans aimed to provide.
Correct Answer: Poverty alleviation
Solution : After the suspension of the planning process, the Sixth Five-year Plan in India focused on poverty alleviation, aiming to improve the living standards of the country's poorest citizens.
Correct Answer: To focus on short-term economic stabilization
Solution : The primary objective of the annual plans implemented during the suspension of the Five-year Plans in India was to focus on short-term economic stabilization, addressing immediate challenges and maintaining continuity in development efforts.
Correct Answer: Political instability
Solution : The primary reason for the suspension of the Five-year Plans in India during the late 1960s was political instability, including internal conflicts and challenges faced by the government, which disrupted the planning process.
