Einstein's Explanation Of Photoelectric Effect - A Complete Guide
Albert Einstein, one of the greatest physicists, contributed his inevitable works and proposed many theories in different fields of physics like the special theory of relativity, concepts of the black hole, photoelectric effect, Einstein quantum theory, Einstein-Maxwell equation, many more topics and some other assumptions. His instinct on the theories never fails. In any field, the Albert Einstein equation plays a prominent role and the Einstein photoelectric equation can be considered as the best achievement of his work. In 1921, he received the Nobel Prize in Physics for his work Einstein theory of photoelectric effect.
This Story also Contains
- What is Einstein Theory of Photoelectric Effect?
- Einstein Explanation of Photoelectric effect:
- What did Einstein’s Theory Explain?
- Photoelectric Effect Derivation
- Applications of Photoelectric Effect
- Numerical Problems on Photoelectric Effect
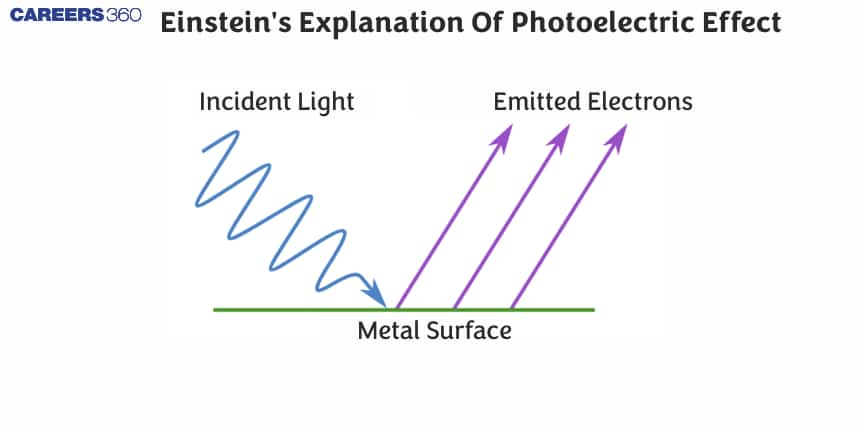
In this article, we will discuss about the Einstein photoelectric equation, derive the equation for the photoelectric effect. We'll also cover important related terms, such as threshold energy, work function, and stopping potential, with related solved numerical examples.
What is Einstein Theory of Photoelectric Effect?
The Einstein theory of photoelectric effect defines that- "Electrons are ejected from the surface of the metal when a ray of light incident on it." These ejected electrons are termed as Photoelectrons. The amount of electrons ejected from the from the surface will depends on the frequency of light incident on it. Let us discuss the explanation of Photoelectric effect.
Einstein Explanation of Photoelectric effect:
Now, let us elaborate and explain the Einstein photoelectric equation as shown in the following diagram:
- The wavy red lines represent light particles that is striking the metal surface. Energy of these photons is determined by Plank's equation:
$E=h \nu$. Where, $h$ is Planck's constant $\left(6.626 \times 10^{-34} \mathrm{Js}\right)$, $\nu$ (or $f$ ) is the frequency of the electromagnetic wave. - When the light particle (photon) hits the metal surface it transfers it energy to the electrons present on the metal surface. If this energy is greater than the work function $(\phi)$ of the metal, then electrons overcome the attractive forces holding it in the metal.
- The electrons will absorb the energy and leave the metal surface, as shown by the arrow directed away from the surface. These arrow represents that the electrons are leaving with Kinetic energy.
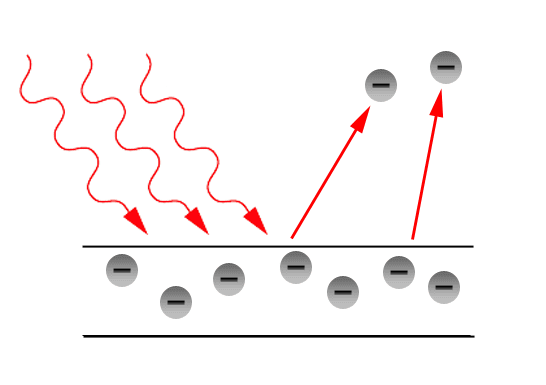
In Brief we can say that the electron will leave the metal surface if the energy provided by the photons will be greater than the work function of the metal.
If the energy provided by the photons are less than the work function, no electron is released from the metal surface.
|
Related Topics |
The laws of Photoelectric Emission:
- There is a minimum cutoff frequency required for emission of electrons. Below this frequency, no electron emission occurs
- The total number of emitted electrons increases with the intensity of incident light
- Kinetic energy of the electrons emitted is dependent on the frequency of light and independent of intensity of light
- There are zero time lags between the incidence of the light beam and electron emission.
Photoelectric Effect Equation
Einstein photoelectric effect equation is given by:
$
\mathrm{KE}_{\max }=h \nu-\phi
$
where:
- $\mathrm{KE}_{\max }$ is the maximum kinetic energy of the ejected electrons,
- $h$ is Planck's constant ( $6.626 \times 10^{-34} \mathrm{~J}$ s),
- $\nu$ (or $f$ ) is the frequency of the incident light,
- $\phi$ is the work function of the metal, which is the minimum energy needed to release an electron from the metal's surface.
Also read -
What did Einstein’s Theory Explain?
Einstein’s theory explained the photoelectric effect, which could not be understood using the wave theory of light.
According to Einstein:
- Light is made of photons (tiny energy packets).
- Each photon has energy E = hν.
A photon can knock out an electron from a metal only if its energy is greater than the work function of the metal.
Photoelectric Effect Derivation
Let us derive Einstein’s photoelectric equation mathematically and this can be written as
Energy of photon = Energy required to remove the electron from the surface of the metal (W) + Maximum Kinetic energy of the electron which is ejected from the surface of the metal.
The energy of the incident photon ( $E=h \nu$ ) is used in two parts:
- To remove the electron from the metal surface, which requires the work function $W$.
- To provide kinetic energy K. E. to the ejected electron.
Therefore, we can write:
$
h \nu=W+\mathrm{K} . \mathrm{E}
$
Rearranging, we get:
$
\mathrm{K} . \mathrm{E} .=h \nu-W
$
At the threshold frequency $\nu_0$, the photon's energy is just enough to overcome the work function without imparting any kinetic energy to the electron. Thus:
$
W=h \nu_0
$
3. Maximum Kinetic Energy:
Substituting $W=h \nu_0$ into the kinetic energy equation, we get:
$
\mathrm{K} . \mathrm{E}_{\cdot \max }=h \nu-h \nu_0
$
or
$
\mathrm{K} . \mathrm{E}_{\cdot \max }=h\left(\nu-\nu_0\right)
$
For an electron with mass $m$ and maximum velocity $v_{\text {max }}$, the kinetic energy can be expressed as:
$
\mathrm{K} . \mathrm{E}_{\max }=\frac{1}{2} m v_{\max }^2=h\left(\nu-\nu_0\right) ----(1) $
The stopping potential $V_0$ is the potential required to bring the ejected electrons to a halt.
Thus:
$
e V_0=\frac{1}{2} m v_{\max }^2
$
Substituting from equation (1), we get:
$
e V_0=h\left(\nu-\nu_0\right) ----(2) $
This equation represents the relationship between the stopping potential $V_0$, the frequency of the incident light $\nu$, and the threshold frequency $\nu_0$ :
$
e V_0=h\left(\nu-\nu_0\right)
$
This final expression shows the Einstein photoelectric effect equation, showing how the maximum kinetic energy (or the stopping potential) depends on the frequency of the incident light and the material’s threshold frequency.
Scientific Terms in Photoelectric Effect
Threshold Frequency:
The minimum frequency of the given incident light beam which is required for the emission of electrons from the surface of the metal is known as Threshold frequency.
Work Function:
The minimum energy required in the removal of the electron from the surface of the metal is known as Work Function. It is represented by $\phi$
Stopping potential:
The required potential to stop the emission of the electron from the surface of the metal is known as stopping potential. It is represented by $V_0$
Applications of Photoelectric Effect
- Photoelectric Cells: Used in automatic street lights, light meters, and photo sensors. They convert light energy into electrical energy.
- Solar Cells: Work on the photoelectric and photovoltaic effect to generate electricity from sunlight.
- TV Remote and Other Sensors: Infrared photo detectors used in remotes, automatic doors, and motion sensors operate using the photoelectric effect.
- Burglar and Fire Alarms: Light beams and photo detectors are used to detect movement or flames.
- Photomultiplier Tubes: Used in scientific instruments to detect very weak light signals.
- Astronomy Instruments: Used to measure the brightness of stars and celestial bodies.
- X-ray Photoelectron Spectroscopy (XPS): Used to study surface composition of materials.
Numerical Problems on Photoelectric Effect
1. The threshold frequency for a certain metal is $5 \times 10^{14} \mathrm{~Hz}$. Calculate the work function of the metal. (Use $h=6.626 \times 10^{-34} \mathrm{~J}$ ).
Solution:
1. Use the formula for the work function $(\phi)$ in terms of the threshold frequency $\left(\nu_0\right)$ :
$
\phi=h \nu_0
$
where $h=6.626 \times 10^{-34} \mathrm{~J}_{\mathrm{s}}$ and $\nu_0=5 \times 10^{14} \mathrm{~Hz}$.
2. Substitute the values:
$
\phi=\left(6.626 \times 10^{-34} \mathrm{~J} s\right) \times\left(5 \times 10^{\mathbf{1 4}} \mathrm{Hz}\right)=3.313 \times 10^{-19} \mathrm{~J}
$
3. Convert this to electron volts ( eV ) by dividing by the charge of an electron, $e=1.6 \times 10^{-19} \mathrm{C}$ :
$
\phi=\frac{3.313 \times 10^{-19} \mathrm{~J}}{1.6 \times 10^{-19} \mathrm{~J} / \mathrm{eV}}=2.07 \mathrm{eV}
$
Answer: The work function of the metal is 2.07 eV .
2- Light of wavelength 400 nm is incident on a metal surface with a work function of 2.2 eV . Will electrons be ejected from the metal surface? If yes, calculate the maximum kinetic energy of the emitted electrons. (Use $h=6.626 \times 10^{-34} \mathrm{Js}^{-}, c=3 \times 10^8 \mathrm{~m} / \mathrm{s}$ ).
Solution:
1. First, calculate the energy of the incident photon using $E=\frac{h c}{\lambda}$ :
$
\begin{aligned}
E & =\frac{\left(6.626 \times 10^{-34} \mathrm{~J}\right) \times\left(3 \times 10^8 \mathrm{~m} / \mathrm{s}\right)}{400 \times 10^{-9} \mathrm{~m}} \\
E & =\frac{1.9878 \times 10^{-25} \mathrm{~J}}{4 \times 10^{-7} \mathrm{~m}}=4.97 \times 10^{-19} \mathrm{~J}
\end{aligned}
$
2. Convert $E$ to electron volts:
$
E=\frac{4.97 \times 10^{-19} \mathrm{~J}}{1.6 \times 10^{-19} \mathrm{~J} / \mathrm{eV}}=3.1 \mathrm{eV}
$
3. Since the photon's energy ( 3.1 eV ) is greater than the work function ( 2.2 eV ), electrons will be ejected from the metal surface.
4. Calculate the maximum kinetic energy of the ejected electrons using the photoelectric equation:
$
\mathrm{KE}_{\max }=E-\phi=3.1 \mathrm{eV}-2.2 \mathrm{eV}=0.9 \mathrm{eV}
$
Answer: Yes, electrons will be ejected, and the maximum kinetic energy of the emitted electrons is 0.9 eV .
Frequently Asked Questions (FAQs)
The threshold frequency formula can be written as W= hνo, where h is Planck’s constant and h = 6.626 x 10-34 J Hz-1
There is a minimum cutoff frequency required for emission of electrons. Below this frequency, no electron emission occurs
The total number of emitted electrons increases with the intensity of incident light
Kinetic energy of the electrons emitted is dependent on the frequency of light and independent of intensity of light
There are zero time lags between the incidence of the light beam and electron emission.
The Photoelectric effect can be explained by assuming that light is incident on the surface of the metal, and then the electron in the surface of the metal gets ejected. This phenomenon of photoelectric effect was explained by the Albert Einstein Photoelectric equation.
The photoelectric effect was initially explained by Heinrich Hertz in the year of 1887 and later it was preceded by Lenard in the year of 1902.
The Einstein photoelectric equation can be written as follows
Eνo= ½ (mvmax2) = h (ν - νo)
