Law Of Thermal Conductivity
Have you ever noticed how quickly a metal spoon is heated up when put in some hot soup, compared to a wooden one? This is caused by a property describing how well a given material conducts heat; it's called thermal conductivity. Understanding the law of thermal conductivity permits us to explain why different materials warm up at different rates and how we can make use of these properties in things like cooking, building homes, or electronic devices.
This Story also Contains
- Law of Thermal Conductivity Law Of Thermal Conductivity
- Solved Examples Based On Law Of Thermal Conductivity
- Summary
In this article, we will cover the concept of the Law Of Thermal Conductivity. This concept falls under the broader category of Properties of Solids and Liquids which is a crucial chapter in Class 11 physics. It is an important topic, last few years many questions asked about this topic in exams like JEE and NEET.
Law of Thermal Conductivity Law Of Thermal Conductivity

Consider a rod of length ' T ', an area of cross-section 'A' whose faces are maintained at temperature $\theta_1$ and $\theta_2$ respectively. In a steady state, the amount of heat flowing from one face to the other face in time t is given by -
$
Q=\frac{K A\left(\theta_1-\theta_2\right) t}{l}
$
$Q=$ Amount of heat transfer
$t=$ Time of heat flow
$K=$ Thermal conductivity of the material
So, from the above equation we can calculate the - Rate of flow of heat i.e. heat current which can be written as -
$
\frac{Q}{t}=H=\frac{K A\left(\theta_1-\theta_2\right)}{l}
$
In the differential form, this heat current can also be written as -
$
\frac{d Q}{d t}=-K A \frac{d \theta}{d x}
$
In the case of a non-steady state or variable cross-section, this is the more general equation that can be used to solve problems.
Relation of Thermal Conductivity of Some Material
$\begin{aligned} & K_{A g}>K_{C u}>K_{A l} \\ & K_{\text {Sold }}>K_{\text {Liquid }}>K_{\text {Gas }} \\ & K_{\text {Metals }}>K_{\text {Non-metals }}\end{aligned}$
Thermal Resistance
$\left(\mathbf{R}_{\mathrm{th}}\right)$: The thermal resistance of a body is defined as the measure of its opposition to the flow of heat through it. It is defined as the ratio of temperature difference to the heat current $\left(\frac{Q}{t}\right)$.
$
R_{t h}=\frac{\theta_1-\theta_2}{H}=\frac{\theta_1-\theta_2}{K A\left(\theta_1-\theta_2\right) / l}=\frac{l}{K A}
$
Recommended Topic Video
Solved Examples Based On Law Of Thermal Conductivity
Example 1: A long metallic bar is carrying heat from one of its ends to the other end under steady-state. The variation of temperature $\theta$ along the length $x$ of the bar from its hot end is best described by which of the following figures?
1)
2) 
3)
4)
Solution:
The heat Flow rate is given by
$
\begin{aligned}
& \frac{d \theta}{d t}=\frac{K A\left(\theta_1-\theta\right)}{x} \Rightarrow \theta_1-\theta=\frac{x}{K A} \cdot \frac{d \theta}{d t} \\
& \theta=\theta_1-\frac{x}{K A} \cdot\left(\frac{d \theta}{d t}\right) \\
& \text { or } \\
& \theta_1=\text { temperature of the hot end }
\end{aligned}
$
and
$\theta=$ temperature at distance x from hot end
$\theta \rightarrow x$ is a straight line with a -ve slope
Hence, the answer is the option (2).
Example 2: Two thin metallic spherical shells of radii $r_1$ and $r_2\left(r_1<r_2\right)$ are placed with their centres coinciding. A material of thermal conductivity K is filled in the space between the shells. The inner shell is maintained at temperature $\theta_1$ and the outer shell at temperature $\theta_2\left(\theta_1<\theta_2\right) \cdot$ The rate at which heat flows radially through the material is :
1) $\frac{\mathrm{K}\left(\theta_2-\theta_1\right)}{\mathrm{r}_2-\mathrm{r}_1}$
2) $\frac{\mathrm{K}\left(\theta_2-\theta_1\right)\left(\mathrm{r}_2-\mathrm{r}_1\right)}{4 \pi \mathrm{r}_1 \mathrm{r}_2}$
3) $\frac{4 \pi \mathrm{Kr}_1 \mathrm{r}_2\left(\theta_1-\theta_2\right)}{\mathrm{r}_2-\mathrm{r}_1}$
4) $\frac{\pi r_1 r_2\left(\theta_2-\theta_1\right)}{r_2-r_1}$
Solution:

$\begin{aligned} & H=\frac{K\left(4 \pi x^2\right)}{d x}(d \theta) \\ & \frac{H}{4 \pi K} \int_{r_1}^{r_2} \frac{d x}{x^2}=\int_{\theta_2}^{\theta_1} d \theta \\ & \frac{H}{4 \pi K} \times\left[-\frac{1}{x}\right]_{r_1}^{r_2}=[\theta]_{\theta_2}^{\theta_1} \\ & \frac{H}{4 \pi K}\left[\frac{-1}{r_2}+\frac{1}{r_1}\right]=\left(\theta_2-\theta_1\right) \\ & \frac{H\left(r_2-r_1\right)}{4 \pi K\left(r_1 r_2\right)}=\theta_1-\theta_2 \\ & H=\frac{4 \pi K\left(r_1 r_2\right)\left(\theta_1-\theta_2\right)}{\left(r_2-r_1\right)}\end{aligned}$
Hence, the answer is the option (3).
Example 3: Temperature difference of $120^{\circ} \mathbf{C}$ is maintained between two ends of a uniform rod $A B$ of length 2 L. Another bent rod $P Q$, of the same cross-section as $A B$ and length $\frac{3 L}{2}$, is connected across $A B$ (See figure). In steady state, the temperature $\left(\right.$ in $\left.{ }^0 \mathrm{C}\right)$ difference between P and Q will be close to :

1) 45
2) 75
3) 60
4) 35
Solution:
Heat Current
$
I_H=\frac{\theta_1-\theta_2}{R_{t h}}
$
where the Temperature difference between two ends of the conductor.
Thermal current =
$
\begin{aligned}
& \frac{\theta_A-\theta_B}{R_{e q}} \\
\text { Thermal current }= & \frac{\theta_A-\theta_B}{\frac{8 R}{5}}=\frac{5 \times 120}{8 \times R}=\frac{75}{R}
\end{aligned}
$
$\begin{aligned} & \theta_p-\theta_Q=\frac{75}{R} \cdot \frac{3 R}{5} \\ & =45^{\circ} \mathrm{C}\end{aligned}$
Hence, the answer is the option (1).
Example 4: Two plates $A$ and $B$ have thermal conductivities $\underline{84} \mathrm{Wm}^{-1} \mathrm{~K}^{-1}$ and $126 \mathrm{Wm}^{-1} \mathrm{~K}^{-1}$ respectively. They have the same surface area and same thickness. They are placed in contact along their surfaces. If the temperatures of the outer surfaces of A and B are kept at $100^{\circ} \mathrm{C}$ and $0^{\circ} \mathrm{C}$ respectively, then the temperature of the surface of contact in steady state is $\qquad$ ${ }^{\circ} \mathrm{C}$.
1) 40
2) 50
3) 20
4) 35
Solution:
$\begin{aligned} & \frac{K_A A\left(T_A-T\right)}{L}=\frac{K_{\mathrm{B}} A\left(T-T_B\right)}{L} \\ & 84(100-T)=126(T-0) \\ & T=40^{\circ} \mathrm{C}\end{aligned}$
Hence, the answer is the option (1).
Example 5: Three rods are arranged in series combination with their length , area and conductivity given in the figure , then their thermal resistance is
1) $\frac{15 l}{6 K A}$
2) $\frac{19 l}{6 K A}$
3) $\frac{23 l}{6 K A}$
4) $\frac{31 l}{6 K A}$
Solution:
In Series Combination
$R_{e q}=R_1+R_2+R_3-\cdots-\cdots-\cdots R_n$
wherein
Req= equivalent thermal resistance
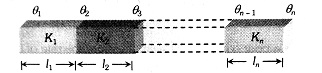
$\begin{aligned} & R=R_1+R_2+R_3 \\ = & \frac{l_1}{K_1 A_1}+\frac{l_2}{K_2 A_2}+\frac{l_3}{K_3 A_3}=\frac{l}{K A}+\frac{2 l}{K A / 2}+\frac{l}{6 K A} \\ & \frac{6 l+24 l+l}{6 K A}=\frac{31 l}{6 K A}\end{aligned}$
Hence, the answer is the option (4).
Summary
The law of thermal conductivity states that the rate of heat transfer through a material is directly proportional to the temperature difference across the material and the area through which the heat is transferred, inversely proportional to the thickness of the material. This law explains the efficiency of heat movement through varied materials. High thermal conductivity will mean quick heat transfers; low thermal conductivity implies slow heat transfers. This principle has generated interest in the design of insulation and heat management in many applications.


