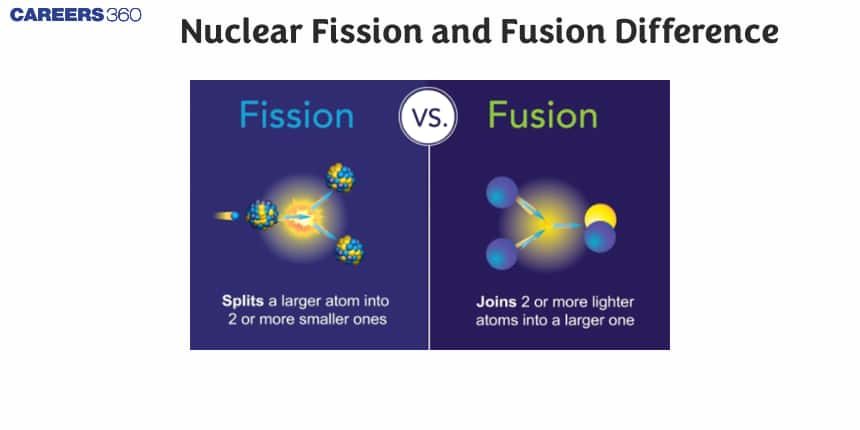
Nuclear Fission and Fusion Difference - A Complete Guide
A type of reaction where two or more atomic nuclei combine to form a different atomic nucleus is called nuclear fusion, while Nuclear fission is the process where heavier items get split into smaller nuclei. The fission process releases a very large amount of energy and photons. Nuclear fusion produces nuclei heavier than the parent nuclei and releases energy upon their formation. Upon the fusion of lighter nuclei, they release energy therefore it is an exothermic reaction. When the fusion of heavier nuclei takes place, energy is accepted so the resulting response can be called endothermic. Nuclear fission and fusion are two chemical processes where a tremendous amount of energy is released. In this article, we will discuss what is nuclear fission and fusion, what is a nucleus, the disadvantages and advantages of nuclear fission and fusion, the difference between nuclear fission and nuclear fusion, and a summary of nuclear fission and fusion in a table.
This Story also Contains
- What is a Nucleus
- What is Nuclear Fission
- What is Nuclear Fusion
- Advantages of Nuclear Fission and Nuclear Fusion
- Disadvantages of Nuclear Fission and Nuclear Fusion
- Difference Between Nuclear Fission and Nuclear Fusion
- Summary of Difference Between Nuclear Fission and Fusion in Table
What is a Nucleus
A nucleus is made up of protons and neutrons and is the basis of nuclear science. Both fission and fusion involve the dispersal and the combination of the nucleus so these two processes are a part of nuclear science. A very important term associated with the fission and fusion process is nuclear binding energy and it is the energy that is required to keep the neutrons and protons in a nucleus. The total mass of individual protons and neutrons is always greater than the mass of elements in the nucleus and the difference in mass can be defined with the help of nuclear binding energy. So nuclear binding energy can also be defined as the missing mass or mass defect or can also be referred to as the mass released.
What is Nuclear Fission
The splitting up of a heavier nucleus into lighter nuclei is called nuclear fission. Nuclear fission was discovered by the German scientists Hahn, Lise Meitner, and Fritz Strassmann in 1938. By bombarding uranium with neutrons a new element that is a lighter element such as barium was formed after the reaction. The following image shows the formation of barium from the uranium-235 ($\mathrm{U}_{92}^{235}$) binary fission process.
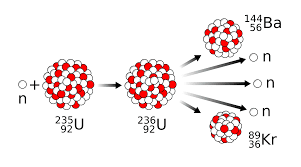
By observing the resulting product we got that nucleus divided asymmetrically. The product obtained varies with every reaction condition. And by observing the reaction more than one neutron is produced as a byproduct. These neutrons are produced as a byproduct and can also induce another fission reaction where a nuclear chain reaction is formed. This means that the fission of $\mathrm{U}_{92}^{235}$ releases three neutrons per fission so this neutron can also be absorbed by other $\mathrm{U}_{92}^{235}$ nuclei and the rate of fission will increase rapidly. A particular mass is required for performing this reaction which means that if the mass of neutrons is low it will escape and cannot be captured for inducing an efficient reaction and the minimum mass required for a sustainable fission reaction is called the critical mass. The enormous amount of energy released during the nuclear chain reaction has many applications in the industrial field.
|
Related Topics, |
What is Nuclear Fusion
The reaction where two nuclei are combined to form a heavier one is called a nuclear fusion and a stable nucleus is formed. This type of reaction is just the opposite of the nuclear fission reaction. When two nuclei that are participating in a reaction have enough kinetic energy to overcome their electrostatic repulsion they will fuse and a nuclear fusion reaction will occur. Fusion reactions are highly exothermic for a lighter nucleus. Let us take some of the nuclear fusion reactions where two deuterium atoms combine to form helium with mass number 3 it is also called deuterium-deuterium fusion. The below reaction shows the formation of helium from the material deuterium fusion. Deuterium and tritium atoms undergo fusion reactions to produce the same helium atom but with the mass number 4. The hydrogen bomb is an example of a fusion reaction and it creates a very high temperature. The nuclear fusion reaction is the power source for stars including the Sun. The following image shows a nuclear fission reaction.
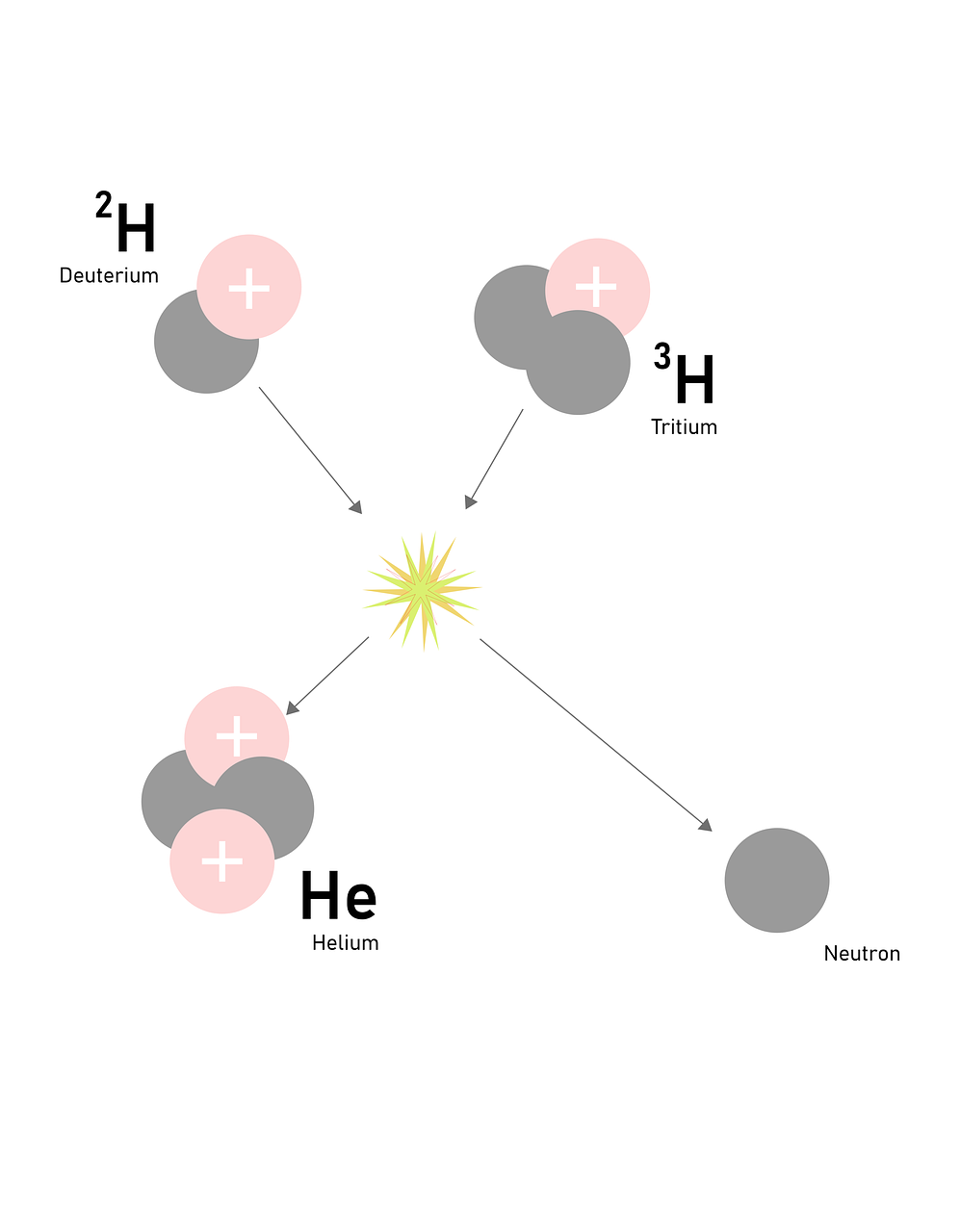
For determining the energy released upon Nuclear fission and fusion reaction we use Einstein's equation which is
$$\mathrm{E}=\mathrm{mc}^2$$
where,
- $m$ is mass in $Kg$
- $c$ is the speed of light
- $E$ is the energy expressed in joules
Advantages of Nuclear Fission and Nuclear Fusion
Nuclear Fission
- No emission of greenhouse gas
- Long-term energy source
- Used in nuclear reactors
- High energy output
- Used for electricity generation
Nuclear Fusion
- Abundant fuel supply
- Highly Efficient
- Produces less radioactive waste
- No greenhouse gas emission
- Provides sustainable energy
Disadvantages of Nuclear Fission and Nuclear Fusion
Nuclear Fission
- Radioactive waste
- Risk of catastrophic failures
- High initial cost
- Thermal pollution
- Construction duration is long
Nuclear Fusion
- More energy is required to initiate the reaction than the energy produced.
- High initial costs
- Complex design
- Produces neutron radiation
- Limited availability of tritium
Difference Between Nuclear Fission and Nuclear Fusion
The very important difference between fission and fusion reaction is that fusion is the combination of lighter nuclei to form a heavier one while fission is the splitting of heavier nuclei to form lighter nuclei. Both fission and fusion involve the release of a tremendous amount of energy. A nuclear fission reaction occurs when a heavier nucleus is bombarded with low-energy neutrons which will further split into lighter nuclei. Nuclear fission reactions are used in nuclear power reactors and nuclear weapons to release a tremendous amount of energy. Both of them released a tremendous amount of energy but the energy released after a nuclear fusion reaction is much greater than nuclear fission. We haven't seen any naturally occurring fission reactions, only fusion reactions that are present in the stars and sun. For initiating a nuclear fission reaction a small amount of energy is needed while for a fusion reaction, large energy is needed because it involves the fusion of two or more nuclei. All the atomic bombs work on the principle of nuclear fission while the hydrogen bomb works on nuclear fusion. Lighter elements like helium and hydrogen are more suitable for a fusion reaction while heavier elements like uranium, thorium, and plutonium are more suitable for a fissionable reaction.
Summary of Difference Between Nuclear Fission and Fusion in Table
| Nuclear Fission | Nuclear Fusion |
| Splitting of a heavy nucleus into smaller nuclei. | Combining of two light nuclei to form a heavier nucleus. |
| Produces significant energy but less than fusion. | Produces much more energy compared to fission. |
| Uranium-235 and plutonium-239 are used as fuel | Hydrogen isotopes like deuterium and tritium are used as fuel |
| Radioactive waste with long half-lives is the byproduct | Helium (non-radioactive) and minimal short-lived waste are the byproducts |
| Moderate temperature is required | Extremely high temperature is required |
| Risk of meltdowns and chain reactions. | Safer, as reactions stop without extreme conditions. |
| Generates radioactive waste; no greenhouse gases. | Minimal waste and no greenhouse gases. |
| Well-developed and in use. | Still experimental, with ongoing research and development. |
Also read:
Frequently Asked Questions (FAQs)
Both Nuclear fission and fusion are used for the production of a tremendous amount of energy but there are some differences between them too. The division of a single nucleus into smaller nuclei is nuclear fission while the combining of two or more small nuclei to form a heavier one is called nuclear fission. A nuclear fission reaction to initiate small energy is required for a nuclear fusion reaction; a large amount of energy is required to combine these small nuclei for the formation of a heavier one. The amount of energy released during a nuclear fusion reaction is very much higher than that of a nuclear fission reaction.
The reactions that are used for the production of a tremendous amount of energy is called nuclear fission and fusion reaction. Fission and fusion reactions are different since for a fission reaction to proceed it requires a very small amount of energy and with that energy, heavier nuclei are split into lighter nuclei with the release of energy. A very common example of nuclear fission is the splitting of Uranium-235 for the formation of barium. While a nuclear fusion reaction is a reaction where the required a large amount of energy to fuse the small nuclei and thereby forming a large nucleus with a tremendous release of energy. A very common example of a nuclear fusion reaction is the combination of deuterium and tritium for the formation of helium with the mass number 4.
Both nuclear fission and fusion involve energy release but there is some difference too.
For fission, it requires small energy while fusion requires a large amount of energy.
The energy released after fusion is higher than that of a fission reaction.
Nuclear fission means the splitting of atomic nuclei into smaller units whereas nuclear fusion is the binding of nuclei together.
Example for nuclear fission: Uranium atom splits into xenon, iodine, barium, etc.
Example for nuclear fusion: A Hydrogen nucleus fuses to form helium.
Nuclear fission is explained by the liquid drop model.
