Temperature - Definition, Example, Unit, FAQs
Introduction
In this article our focus will be on temperature, temperature definition, what is the temperature, temperature meaning, types of temperature etc.

What is the temperature? Or define Temperature. Or What is the definition of temperature?
Temperature is a measure of hotness or coldness expressed on any of numerous arbitrary scales that indicates the direction in which heat energy would naturally flow—that is, from a hotter (higher) body to a colder (lower) body (one at a lower temperature). Temperature is not the same as energy in a thermodynamic system; for example, a burning match has a significantly higher temperature than an iceberg, but the total heat energy in an iceberg is far greater than the energy in a match. Temperature, like pressure or density, is referred to as an intensive property—one that is unaffected by the amount of stuff being considered—as distinguished from extensive properties, such as mass or volume
Mainly three temperature scales are widely used. Out of them, the Fahrenheit (°F) temperature scale and Celsius (°C) temperature scale is widely used in science and is used practically everywhere. The Kelvin (K) scale is the international standard for measuring temperature in scientific applications.
In some technological specialties, a distinct absolute temperature scale, the Rankine scale (see William Rankine), is chosen over the Kelvin scale. The kelvin equals one Celsius degree, while the degree Rankine (°R) equals one Fahrenheit degree.
In the 18th and 19th centuries, the Réaumur (°Re) temperature scale (or octogesima division) was commonly employed in areas of Europe; it was afterwards largely used to measure the temperature of combinations during brewing, syrups in the preparation of specific culinary products, and milk during cheese production.
Also read -
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 12 Physics
- NCERT Solutions for All Subjects
What do you mean by temperature? Or Temperature Definition Physics
Temperature is a term used in physics to describe the physical properties of matter that quantify the hotness or coldness of a body. We used to judge temperature based on human perception - whether an object was hot or cool was largely judged by human touch before the formation of the idea of temperature. This, however, is not correct. A wooden table, for example, may appear warmer than a metal cycle rod on a cold morning. Both, however, have the same temperature due to the external environment. Metal, as a greater conductor of heat, pulls heat away from your body faster than wood, making it cooler (a poor conductor of heat). The physical quantity measured with a thermometer is a simple temperature definition in science. The kinetic energy of the molecules and atoms inside an item, on the other hand, are intimately connected to temperature.
Temperature and Kinetic Energy
According to physics, an object's kinetic energy is the energy it has as a result of its motion. Kinetic energy exists in every molecule or atom. Even when molecules are closely packed in solids, they all have vibrational energy. The average kinetic energy of all molecules is referred to as temperature. When heat is absorbed by a substance, the molecules begin to move quicker. This raises the kinetic energy of the object. As a result, the substance's temperature begins to rise. The movement of molecules can eventually lead to their separation as they move away from one another. When the temperature of a solid rises, for example, the molecules begin to move quicker, causing the solid to expand. If the temperature climbs to the melting point of the solid, it will eventually change state. Heat is the transfer of energy from an object having higher temperature to an object having lower temperature.
Example of Temperature
Many people mistake temperature for energy, which is not the case. Temperature is just a measurement of one atom or molecule's average kinetic energy. Temperature and humidity are displayed in two columns during weather reports. Because of human perception rather than temperature changes, hot and humid days are perceived as hotter than hot and dry days. If the temperature is the same but the humidity is high, a person may feel hotter because sweat does not drain as quickly on humid days as it does on dry days. Sweat evaporates, allowing us to stay cooler
What is the Absolute Zero?
Absolute zero is the coldest temperature on the planet. 0 Kelvin, or -273.15 degrees Celsius, or -460 degrees Fahrenheit, is the temperature. There is no heat energy in a substance at absolute zero temperature. At this temperature, the particles in a perfect crystal will be immobile, which means that their kinetic energy will be zero. Although absolute zero is theoretically possible, humans have yet to see absolute zero temperatures in practice. Using cryocoolers and dilution refrigerators, we have been able to get near to absolute zero.
Related Topics Link, |
Units of Temperature
Temperature has units to express it since it is a physical quantity. The SI unit of temperature is Kelvin. However, we also use the Celsius and Fahrenheit units to measure temperature. We know,
![]()
![]()
![]()
How do Thermometers Measure Temperature?
Thermometers are commonly used to determine a person's temperature. A temperature sensor, such as the bulb of a mercury-in-glass thermometer, and a numerical scale are the two most important components of a thermometer. The principle of thermal expansion governs the operation of a mercury thermometer. The change in volume of a substance caused by a change in temperature is known as thermal expansion. Mercury is utilized in thermometers because it is sensitive to temperature fluctuations. As the mercury inside the thermometer expands and climbs upwards, a rise in temperature is soon noted. The temperature is measured using the numeric scale of the thermometer. Celsius thermometers, Fahrenheit thermometers, and Kelvin thermometers are the three types of thermometers.
Also read :
- NCERT solutions for Class 11 Physics Chapter 11 Thermal Properties of Matter
- NCERT exemplar class 11 physics solutions chapter 11 Thermal Properties of Matter
- NCERT notes Class 11 Physics Chapter 11 Thermal Properties of Matter
Temperature Scales
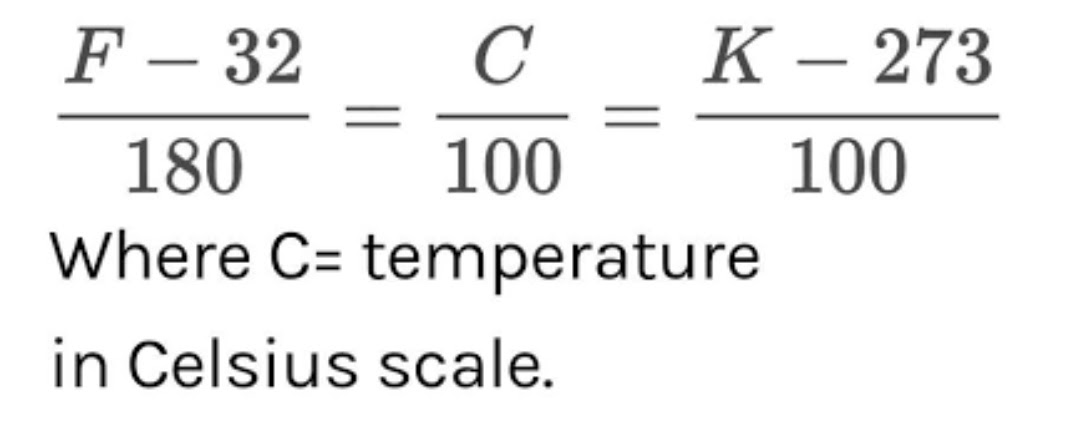
Because thermometers must be of a certain length, each one has a fixed scale. Because the fixed scale used for Celsius thermometers is set at the freezing and boiling points of water, they are simple to make. The lower fixed point of a Celsius thermometer is 0°C (the freezing point of water) and the upper fixed point is 100°C (boiling point of water). The lower fixed point on a Fahrenheit thermometer is 32 degrees Fahrenheit, while the upper fixed point is 212 degrees Fahrenheit. The lower fixed point of a Kelvin thermometer is 273 degrees Celsius, and the upper fixed point is 373 degrees Celsius. We may also deduce the relationship between Celsius, Fahrenheit, and Kelvin from these fixed points.
Also check-
- NCERT Exemplar Class 11th Physics Solutions
- NCERT Exemplar Class 12th Physics Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Physics Notes :
Frequently Asked Questions (FAQs)
Temperature is a measure of hotness or coldness expressed on any of numerous arbitrary scales that indicates the direction in which heat energy would naturally flow—that is, from a hotter (higher) body to a colder (lower) body (one at a lower temperature). Temperature is not the same as energy in a thermodynamic system; for example, a burning match has a significantly higher temperature than an iceberg, but the total heat energy in an iceberg is far greater than the energy in a match. Temperature, like pressure or density, is referred to as an intensive property—one that is unaffected by the amount of stuff being considered—as distinguished from extensive properties, such as mass or volume.
Temperature is a term used in physics to describe the physical properties of matter that quantify the hotness or coldness of a body. We used to judge temperature based on human perception - whether an object was hot or cool was largely judged by human touch before the formation of the idea of temperature. This, however, is not correct. A wooden table, for example, may appear warmer than a metal cycle rod on a cold morning. Both, however, have the same temperature due to the external environment. Metal, as a greater conductor of heat, pulls heat away from your body faster than wood, making it cooler (a poor conductor of heat). The physical quantity measured with a thermometer is a simple temperature definition in science. The kinetic energy of the molecules and atoms inside an item, on the other hand, are intimately connected to temperature.
Mainly three temperature scales are widely used. Out of them, the Fahrenheit (°F) temperature scale and Celsius (°C) temperature scale is widely used in science and is used practically everywhere. The Kelvin (K) scale is the international standard for measuring temperature in scientific applications.
In some technological specialties, a distinct absolute temperature scale, the Rankine scale (see William Rankine), is chosen over the Kelvin scale. The kelvin equals one Celsius degree, while the degree Rankine (°R) equals one Fahrenheit degree.
The ability of your body to produce and remove heat is measured by its temperature. Even when the temperature outside the body fluctuates a lot, the body is very good at maintaining a safe temperature range. A person's typical body temperature is 37 degrees Celsius or 98.6 degrees Fahrenheit.
During a certain time period, usually a day, month, or year, the average temperature of the air as measured by a suitably exposed thermometer.
Average kinetic energy of an object is measured by the temperature.
Thermometer is used to measure the temperature.
Daniel Fahrenheit
The average temperature is 14 degree celsius.
Questions related to
On Question asked by student community
Hello aspirant,
Natural elements like latitude, height, and the existence of ocean currents affect a region's temperature characteristics. Factors like a region's closeness to mountain ranges and predominant winds might affect its precipitation characteristics.
Thank you
Hope it helps you
Correct Answer: wild
Solution : The first option is correct.
The threats mentioned in the passage, such as habitat destruction and conflicts with local people, suggest that the context is about the challenges faced by elephants in their natural, wild habitat. So, wild is the appropriate word in this context.
Correct Answer: vulnerable
Solution : The correct answer is vulnerable.
Majority of the maize production depends on natural rainfall, making it vulnerable to variable rainfall patterns.
Explanation:
Only the word "vulnerable" will do to fill in the blank and make the sentence comprehensible.
It denotes being at risk of attack
Correct Answer: The process occurs at a temperature above 430oC at atmospheric pressure.
Solution : The answer is The process occurs at a temperature above 430oC at atmospheric pressure.
Pyrolysis is a disintegration technology that converts organic biomass into liquids rich in carbon content that can
