Relation Between Kp And Kc - A Complete Guide
kp and kc are equilibrium constants in ideal gas mixture. KP is the equilibrium constant taken with respect to atmospheric pressure and kc is the equilibrium constant used to express the concentration of gaseous mixture in terms of molarity.
Let us consider an equilibrium state in a gaseous mixture of reactants A , B and products C ,D .
Also read -
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 12 Physics
- NCERT Solutions for All Subjects
The equation of the reaction be ,
aA + bB⇌cC+dD
In the above equation a, b, c and d are the mole numbers for A, B, C, and D respectively.
Therefore, the kc can be written as
![k_c=\frac{[c]^d[D]^d}{[A]^a[B^b]}](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650281372682.png)
So, the kp can be written as
![kp=\frac{[p_c]^c[p_o]^d}{[p_A]^a [p_B]^b}](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650281740782.png)
Here , pA,pB,pC and pD are the partial pressure of the gas A,B,C and D respectively.
Also Read:
- NCERT solutions for Class 11 Physics Chapter 13 Kinetic Theory
- NCERT Exemplar Class 11 Physics Solutions Chapter 13 Kinetic Theory
- NCERT notes Class 11 Physics Chapter 13 Kinetic Theory
Example 1 :
2A+3B⇌4C+5D
![k_c=\frac{[C]^4[D]^5}{[A]^2[B]^3}](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650282183700.png)
Example 2 :
2A+3B⇌4C+5D
![k_p=\frac{[P_c]^4[p_D]^5}{[P_A]^2[P_B]^3}](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650282429373.png)
Here , pA,pB,pC and pD are the partial pressure of the gas A,B,C and D respectively.
From the equation of ideal gas ;
pV=nRT
Where , p = pressure ; V = volume ; n = no of moles ; R = gas constant ; T = temperature.
Related Topics Link, |
How to find kp?
Derive the relation between kpand kc/Write kp kc relation/write the relation between kp and kc
Therefore,
pV=nRT
Or, 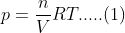
From the above equation we can say;
pA=[A]RT
pB=[B]RT
pC=[C]RT
pD=[D]RT
So, the
![k_p=\frac{[p_c]^c[p_D]^d}{[p_A]^a[p_B]^b}.......(2)](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650283167132.png)
![k_c=\frac{[c]^c[D]^d}{[A]^a[B]^b}.......(3)](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650283264273.png)
Substituting the values of, pA,pB,pC and pD in the equation (2)
![k_p=\frac{[c]^c [RT]^c[D]^d [RT]^d}{ [A]^a [RT]^a[B]^b[RT]^b}](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650283798898.png)
![k_p=\frac{[c]^c [D]^d[RT]^{(c+d)}} { [A]^a [B]^b[RT]^{(a+b)}}](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650284538943.png)
![k_p=\frac{[c]^c [D]^d[RT]^{(c+d){(a+b)})}} { [A]^a [B]^b](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650285177932.png)
![k_p=\frac{[c]^c[D]^d}{[A]^a[B]^b}[RT]^{\Delta n}.........(4)](https://cache.careers360.mobi/media/articles/uploads/froala_editor/images/2022/4/18/1650286002108.png)
Here, (c+d) is the sum of the mole number of the products and (a+b) is the sum of the mole number of the reactants.
So, ∆n is the difference between the sum of the mole number of the products and the sum of the mole number of the reactants.
∆n=(c+d)-(a+b)
Substituting the value of equation (3) in equation (4):
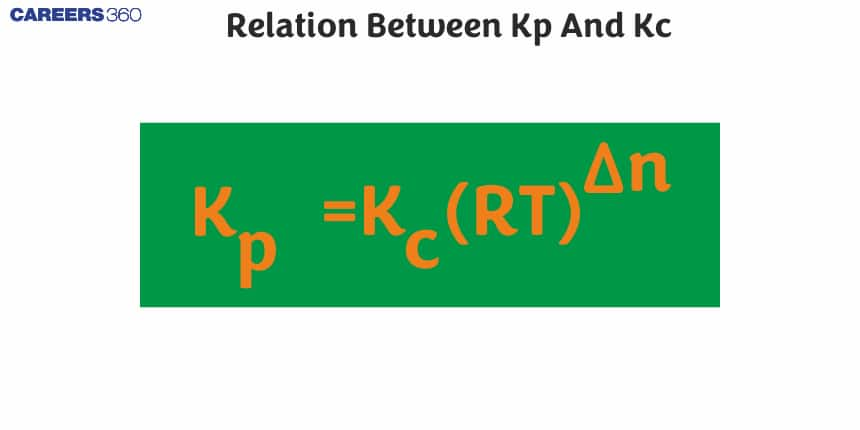
Then the the relation between kp and kc is,
kp=kc[RT]∆n
The above equation is the relation between kp and kc.
Special case, ∆n=0 then kp=kc ; it happens when there is no change in the mole numbers between the sum of the mole number of the products and the sum of the mole number of the reactants.
If , kp=kc[RT]∆n then kc=kp[RT]-∆n is the relation between kc and kp.
These are included in the relation between kp and kc pdf/relation between kp and kc class 11th chemistry.
Also check-
- NCERT Exemplar Class 11th Physics Solutions
- NCERT Exemplar Class 12th Physics Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Physics Notes:
Frequently Asked Questions (FAQs)
Kp constant of equilibrium w.r.t. the atmospheric pressure and Kc is the constant of equilibrium w.r.t. the molar concentration of the gas mixture.
Kx depends on the atmospheric pressure while Kp and Kc are independent of the pressure.
Ka and Kb determine the dissociation property of acid-base.
Kp is the constant of equilibrium applicable in the partial pressures while Kc is the constant of equilibrium applicable in the concentrations.
