Boltzmann Constant - Definition, Formula, Value, FAQs
The Boltzmann constant is a proportionality constant that is associated with the relative average kinetic energy of the particles present inside the gas with the gas’s Thermodynamics temperature. It happens in the description of the gas constant in Planck's ordinance of Black body radiation and the formula of Boltzmann entropy and in the kelvin scale. It was the Austrian researcher Ludwig Boltzmann, who came up with this constant formula, and thus was given the name Boltzmann Constant, consisting of dimensions similar to the entropy which is energy divided by the temperature.
This Story also Contains
- History of the Invention of the Boltzmann Constant
- What is the Boltzmann Constant?
- The Formula of Boltzmann Constant
- Value Of Boltzmann Constant
- Relation Between Boltzmann Constant and Gas Constant
- Boltzmann Constant Applications
- Summary
The Boltzmann constant is one of the most well-received seven defining constants which has applications in different mergers to describe the SI base units of the seven defining constants.
History of the Invention of the Boltzmann Constant
The name Boltzmann constant was given the name after the Austrian researcher, Ludwig Boltzmann in the 19th century. Even though it was Ludwig first in the year 1877, who associated probability and entropy, in the black body radiation derivation initially in the 19th century, however, the association was not at all once indicated with a particular constant until Planck, invented the k and presented the most accurate value for it in the ordinance of the black body radiation derivation in the 19th century initially. Prior to the 19th century, calculations regarding the factors of Boltzmann were not put in writing applying the molecule per energy and the Boltzmann constant, instead applying a gas constant form, R and energy in macroscopy and quantities in macroscopy of the compound.
In the year 2017, the most precise calculations of the Boltzmann constant were acquired by gas thermometry which is acoustic, which represents the velocity of the volume of the monatomic gas in a chamber which is triaxial ellipsoid with applying resonances of acoustic and microwave. This time-consuming exertion was accepted with various experiments by many laboratories. It is one of the most well-received cornerstones of the year 2019 SI base unit redefinition. Based upon the calculations, the CODATA endorsed 1.380 649 × 10-23 J.K-1 to be the concluding Boltzmann constant value to be applied for the international system of the units.
What is the Boltzmann Constant?
The Boltzmann constant is a fundamental physical constant that relates the average kinetic energy of particles in a gas to the temperature of the gas. It acts as a bridge between the microscopic world (atoms and molecules) and the macroscopic world (temperature and energy).
It is denoted by the symbol $k_B$
The Formula of Boltzmann Constant
The formula of the Boltzmann constant relates the gas constant $(\mathbf{R})$ to Avogadro's number $\left(\mathbf{N}_a\right)$. It is given by:
$
k_B=\frac{R}{N_A}
$
where:
- $k_B=$ Boltzmann constant
- $R=$ Universal gas constant $=8.314 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$
- $N_A=$ Avogadro's number $=6.022 \times 10^{23} \mathrm{~mol}^{-1}$
Value Of Boltzmann Constant
The value of the Boltzmann constant is:
$k=1.380649 \times 10^{-23} \mathrm{~J} \mathrm{~K}^{-1}$
Other Units:
In erg per kelvin (CGS system):
- $k=1.380649 \times 10^{-16} \mathrm{erg} \mathrm{~K}^{-1}$
In electronvolt per kelvin ( $\mathrm{eV} / \mathrm{K}$ ):
- $k=8.617333 \times 10^{-5} \mathrm{eV} \mathrm{~K}^{-1}$
Relation Between Boltzmann Constant and Gas Constant
Relation Between Boltzmann Constant and Gas Constant
The Boltzmann constant (k) and the gas constant (R) are related through Avogadro's number (NA).
The relation is given by the formula:
$
R=k \times N_A
$
Where,
- $R=$ Gas constant $=8.314 \mathrm{~J} / \mathrm{mol} \cdot \mathrm{K}$
- $k=$ Boltzmann constant $=1.38 \times 10^{-23} \mathrm{~J} / \mathrm{K}$
- $N_A=$ Avogadro's number $=6.022 \times 10^{23} \mathrm{~mol}^{-1}$
|
Related Topics, |
Boltzmann Constant Applications
The Boltzmann constant is very useful in many areas of physics. Some of its main applications are:
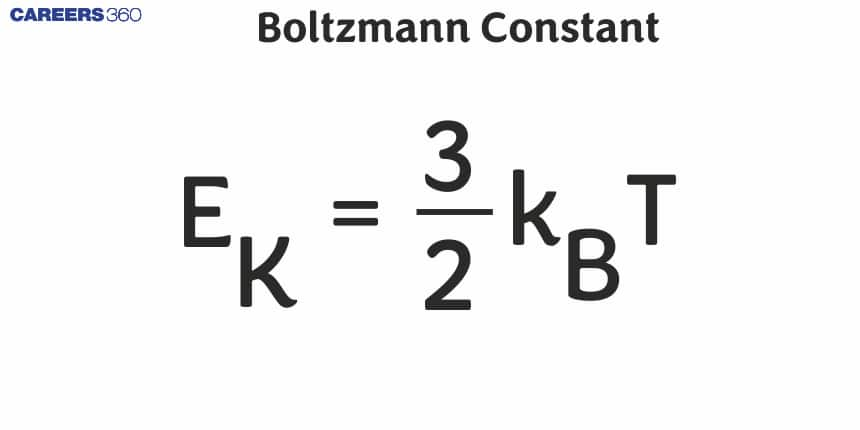
1. Kinetic Theory of Gases:
It relates the average energy of gas molecules to the temperature using the formula $E=\frac{3}{2} k T$.
2. Statistical Mechanics:
It helps to connect the behavior of individual atoms or molecules with the overall properties of matter.
3. Entropy Calculation:
It is used in the formula $S=k \ln W$ to find the entropy of a system.
4. Thermal Noise:
It is used to calculate the heat or noise produced in electrical circuits.
5. Blackbody Radiation:
It helps in studying how objects emit radiation depending on their temperature.
Summary
The Boltzmann constant was named after Ludwig Boltzmann. As the average kinetic energy of gas particles is connected to the temperature, it is a connection between microscopic and macroscopic physics. Defined as $1.380649 \times 10^{-23} \mathrm{~J} / \mathrm{K}$, it forms part of many equations in thermodynamics and is an important tool for understanding gas behaviour, entropy, and black-body radiation.
Frequently Asked Questions (FAQs)
The constant is a physical quantity that is known to be ubiquitous in nature and has a constant numerical value in all the conditions applied.
Boltzmann constant is the constant of the ratio of gas to Avogadro’s number equivalent to the numerical value of 1.381×10-23 joules per kelvin as in the case of where the molecules in existence are equivalent to Avogadro’s number. Boltzmann constant has significance in many branches namely, Planck’s constant, Clausius thermodynamics and is also an interconnected link between microscopic and macroscopic branches of physics.
S= kb log w is the equation for Boltzmann entropy.
In semiconductors, the Shockley diode equation determines the interconnection between the flow rate of electric current and the potential of electrostatic along p and n junction diodes which depends on a particular voltage called the thermal voltage, which is abbreviated as VT. the thermal voltage totally depends on absolute temperature T as
VT = kT/ q where q stands for the magnitude of the electron’s electric charge.
Boltzmann constant k is equal to 228.5991672dB (W/K/Hz)
Boltzmann constant can be expressed in units such as, J/K, eV/K, cal/K, dB, erg/K etc.
K is applied as a coulomb constant in physics which is numerically equivalent to the value of K = 9×109 newton meter square and centimeter square.
